Langrand 1990) Or 15 (Goodman Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Sickle-Billed Vanga Falculea Palliata Belongs to the Vangidae, a Family Endemic to Madagascar (Milon Et Al
山階鳥学誌 (J. Yamashina Inst. Ornithol.), 35: 155-158, 2004 The Diet of Adult and Nestling Sickle-billed Vangas Falculea palliata, a Species Endemic to Madagascar Masahiko Nakamura*, Takayoshi Okamiya* and Satoshi Yamagishi** Abstract. The Sickle-billed Vanga Falculea palliata forages by probing its long and slender bill into cracks on the trunks and branches of trees. To determine the diet of adult and nestling Sickle-billed Vangas and the kinds of prey obtained with this foraging technique, six nests in the Ankarafantsika Strict Nature Reserve, western Madagascar, were observed during the November to December nestling periods in 1999 and 2000. Diet was investigated by direct observation. Of the 41 items of prey identified (out of 68 total prey items) in adult Sickle-billed Vangas, crickets, cockroaches, spiders, and grasshoppers constituted 29.3%, 19.5%, 17.1%, and 12.2%, respectively, of the prey items. These four groups together accounted for 78.0% of the identified prey. Parents delivered 1,180 prey items to the nestlings. The most numerous food items were crickets, accounting for 41.6% of 262 identified prey, followed by cockroaches (21.4%) and grasshoppers (15.6%). These three groups together accounted for 78.6% of the identified prey. Sickle-billed Vangas never foraged on the ground but preferred dead trees of five metres or higher for foraging, and it is thus highly probable that all prey items were of arboreal origin. Arboreal cockroaches inhabit holes and cracks on dead trees and branches, and it is likely that Sickle-billed Vangas capture them primarily using the probing technique. -

Madagascar Highlights I 11 Th to 25 Th July 2011 (15 Days)
Madagascar Highlights I 11 th to 25 th July 2011 (15 days) Trip Report Trip report compiled by tour leader: Rainer Summers Tour Summary Sometimes referred to as the “laboratory of evolution”, Madagascar, the huge Indian Ocean island situated 500km off the coast of east Africa, has long attracted the attention of naturalists and travelling birders alike. Our winter tour, although a departure from the standard summer tours to the “Red Isle”, was very successful, and we managed to see a fantastic proportion of the amazing creatures that call Madagascar home. Trip Report RBT Madagascar Highlights I 2011 2 We began our first day with a visit to the Tsimbazaza Zoo, where despite the overcast weather we managed to find Eleonora’s Falcon, Mascarene Martin and the first of many Madagascar Buzzards, while our afternoon at Lake Alarobia proved to be most enjoyable, with large numbers of waterfowl including Knob-billed Duck and Red-billed Teal, Black and Dimorphic Egret, Malagasy Kingfisher and Madagascar Swamp Warbler, before enjoying a sumptuous dinner at our comfortable accommodations. The eastern rainforests of Madagascar harbour a rich assemblage of sought-after mammals and birds, and for this reason the forested zone in the vicinity of Perinet village formed the basis of our explorations for four days. Our time was divided between visits to the reserve at Perinet and the more distant Mantadia National Park, both offering good rainforest birding. Despite the less than optimal weather during our time in Perinet- Mantadia, our hard work and time in the field paid off, and we were rewarded with a mouth-watering selection of eastern rainforest endemics. -

University of Victoria Department of Biology INCREASES IN
University of Victoria Department of Biology INCREASES IN CHARCOAL PRODUCTION EFFICIENCY AND THE IMPLEMENTATION OF A SUSTAINABLE CHARCOAL SUPPLY CHAIN TO THE CITY OF TOLIARA IN SOUTHWESTERN MADAGASCAR WORK TERM REPORT In partial fulfillment of the requirements of the Biology Co-op Program Winter 2010 Work Term 1 By Julie Bremner WWF Explore International Youth Volunteer Performed at : WWF Madagascar and West Indian Ocean Programme Office Ankilimalinika, Madagascar Job Supervisor : Rina Andrianarivony Fuel wood Project Officer 1 TABLE OF CONTENTS ABSTRACT 2 INTRODUCTION 3 DISCUSSION 8 CONCLUSION 16 WORKS CITED 18 MAPS 20 2 ABSTRACT The Spiny Forest Ecoregion of Southwestern Madagascar is a zone of tremendous biodiversity and endemism. It is of key importance to the subsistence lives of villagers in the region and the urban population of Toliara that increasingly depends on forest fuel wood resources for their daily energy needs. Prolonged drought conditions in the area have led to increasing demands on the forest while villagers switch from farming to charcoal production as a means of earning a living. Urban population growth and resultant fuel wood demand increase has further exacerbated the deforestation of the spiny forest, which is currently exhibiting the highest rate of deforestation in Madagascar. WWF has stepped in to attempt to mitigate future forest loss through the establishment of the Synergy Energy Environment in the South West (SEESO) project. SEESO has as its goal the establishment of a sustainable fuel wood supply chain to the city of Toliara originating from the Atsimo-Andrefana region. The project is encouraging the adoption of a more efficient charcoal production technique, the plantation of trees for future charcoal production and the implementation of a system of regulations and governing bodies that will ensure the prolonged sustainability of the region’s forest resources. -

MADAGASCAR TRIP REPORT Aug.-‐Sept 2012 John Clark
MADAGASCAR TRIP REPORT Aug.-Sept 2012 John Clark ([email protected]) Our London friends, Dick and Liz Turner, Mary Ward-Jackson and I spent almost 4 weeks in Madagascar. Our primary focus was Birds, But we were also interested in nature more Broadly and culture. The tour was excellently prepared By our guide, Fanomezantsoa Andrianirina (Fano) – who was a perfect guide as well as Being great fun to travel with. The trip was excellent and we ended up seeing 122 of the endemic (and endemic Breeding) Birds of Madagascar, plus 54 non-endemics. Fano was not only an excellent Bird-guide himself, But he had lined up local guides in most of the locations – most of whom were terrific (especially, perhaps, Jaqui in Ampijoroa). Fano is doing much to help develop these local guides as more experienced and confident bird-guides in their own right. The logistics and places to stay were excellent – well, as excellent as an inevitaBle dependence on Madagascar Air permits! (They don’t call it Mad. Air for nothing; it is quite the worst airline I have ever had to use!). Fano’s drivers were also terrific (and keen budding birders!) So our main advice, for those planning a birding (or indeed broader nature/wildlife) trip to Mad. is to use Fano if at all possible. He was totally professional, accurate, dogged, scientifically knowledgeaBle about the Bird, mammals and other species and became a good friend. He can Be contacted By email on [email protected], phone: (+261)32 02 017 91 or website: www.madagascar-funtourguide.com If you want more info on the trip, please email me, and if you’d like to see some of our photos go to: https://picasaweb.google.com/104472367063381721824/Madagascar2012?authkey=Gv1sRgcJH0nYK-wenN9AE# Itinerary Aug. -

Reference File
References added since publication of 2007 CRC Handbook of Avian Body Masses Abadie, K. B., J. Pérez Z., and M. Valverde. 2006. Primer reporte de colonias del Martín Peruano Progne murphyi. Cotinga 24:99-101. Ackerman, J. T., J. Y. Takekawa, J. D. Bluso, J. L. Yee, and C. A. Eagles-Smith. 2008. Gender identification of Caspian Terns using external morphology and discriminant function analysis. Wilson Journal of Ornithology 120:378-383. Alarcos, S., C. de la Cruz, E. Solís, J. Valencia, and M. J. García-Baquero. 2007. Sex determination of Iberian Azure-winged Magpies Cyanopica cyanus cooki by discriminant analysis of external measurements. Ringing & Migration 23:211-216. Albayrak, T., A. Besnard, and A. Erdoğan. 2011. Morphometric variation and population relationships of Krüeper’s Nuthatch (Sitta krueperi) in Turkey. Wilson Journal of Ornithology 123:734-740. Aleixo, A., C. E. B. Portes, A. Whittaker, J. D. Weckstein, L. Pedreira Gonzaga, K. J. Zimmer, C. C. Ribas, and J. M. Bates. 2013. Molecular systematics and taxonomic revision of the Curve-billed Scythebill complex (Campylorhamphus procurvoides: Dendrocolaptidae), with description of a new species from western Amazonian Brazil. Pp. 253-257, In: del Hoyo, J., A Elliott, J. Sargatal, and D.A. Christie (eds). Handbook of the birds of the world. Special volume: new species and global index. Lynx Edicions, Barcelona, Spain. Volume 1. Alfano, A. 2014. Pygmy Nightjar (Nyctopolus hirundinaeus). Neotropical Birds Online (T.S. Schulenberg, ed.). Cornell Laboratory of Ornithology, Ithaca, NY. Alvarenga, H. M. F., E. Höfling, and L. F. Silveira. 2002. Notharchus swainsoni (Gray, 1846) é uma espécie válida. -

MADAGASCAR: the Wonders of the “8Th Continent” a Tropical Birding Set Departure
MADAGASCAR: The Wonders of the “8th Continent” A Tropical Birding Set Departure November 3—28, 2013 Guide: Ken Behrens All photos taken during this trip. All photos by Ken Behrens unless noted otherwise. TOUR SUMMARY Madagascar has long been a core destination for Tropical Birding, and with last year’s opening of a satellite office in the country, we have further solidified our expertise in the “Eighth Continent.” This was another highly successful set-departure tour to this special island. It included both the Northwestern Endemics Pre-Trip at the start and the Helmet Vanga extension to the Masoala Peninsula at the end. Although Madagascar poses some logistical challenges, especially in the form of the national airline Air Madagascar, we had no problems on this tour, not even a single delayed flight! The birding was great, with 196 species recorded, including almost all of the island’s endemic birds. As usual, the highlight was seeing all five of the incredible ground-rollers, from the roadrunner-like Long-tailed of the spiny forest to the wonderful rainforest-dwelling Scaly. There was a strong cast of vangas, including Helmet, Bernier’s, and Sickle-billed. In fact, we saw every member of the family save the mysterious Red-tailed Newtonia which is only regularly seen in the far south. As normal, the couas were also a favorite. From the shy and beautiful Red-breasted of Madagascar Set Departure Tour Nov. 3-28, 2013 the eastern rainforest to the huge Giant Coua of the dry western forest, we were looking for and at couas virtually every day! The bizarre mesites form a Malagasy endemic family, and we had superb extended views of all three members of the family. -
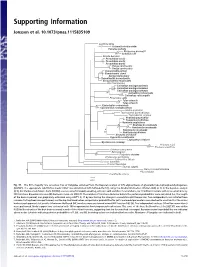
Supporting Information
Supporting Information Jønsson et al. 10.1073/pnas.1115835109 Fig. S1. The 50% majority rule consensus tree of Vangidae obtained from the Bayesian analysis of 375 aligned bases of glyceraldehyde-3-phosphodehydrogenase (GAPDH). The appropriate substitution model TIM+Γ was determined with MrModeltest (1), using the Akaike information criterion (AIC) (2, 3). In the Bayesian analysis (4, 5), the Markov chain Monte Carlo (MCMC) was run using Metropolis coupling, with one cold and three heated chains, for 10 million iterations with trees sampled every 500 iterations. Bayesian inference (BI) harmonic mean −ln 2962.70. The number of iterations discarded before the posterior probabilities were calculated (i.e., the length of the burn-in period) was graphically estimated using AWTY (6, 7) by monitoring the change in cumulative split frequencies. Two independent runs initiated from random starting trees were performed, and the log-likelihood values and posterior probabilities for splits and model parameters were checked to ascertain that the chains had reached apparent stationarity. Maximum-likelihood (ML) analyses were performed using GARLI version 0.95 (8). Five independent analyses (20 million generations) were performed. Nodal support was evaluated with 100 nonparametric bootstrap pseudoreplications. The score of the best-likelihood tree (−ln 2827.81) was within 0.05 likelihood units of the best tree recovered in each of the other four runs, suggesting that the five runs had converged. Bayesian posterior probabilities are indicated above nodes (asterisks indicate posterior probabilities of 1.00), and ML bootstrap values are indicated below nodes. Members of Vangidae are indicated in bold. 1. Nylander JAA (2004) MrModeltest (Uppsala University, Uppsala) (http://www.abc.se/∼nylander), Version 2. -

Birds of Madagascar and Their Conservation
Birds of Madagascar and Their Conservation byMichael S. Putnam Department ofZoology University of Wisconsin tl Madison, Wisconsin I 100 plus Ecstatic Testimonials Striding up a steep hillside with the Council for Bird Preservation (Collar loud whisper of a rushing stream in and Stuart, 1985), 28 species of Warm, nurturing foods. the background, I stepped into a Malagasy birds are threatened and 14 mist-net lane I had cut a week before. species are considered as near Cook monthly, As I entered the clearing, a medium threatened. These species represent freeze in packets, sized brown bird squawked and flew between one-fifth and one-third of off from eye-level. Carefully search the island's endemic bird species. serve in seconds. ing the nearby vegetation, I became one of a lucky handful of foreigners The primary threats to Madagas At fine stores, orcall... to ever find a nest of the Brown car's birds today, habitat loss and Mesite (Mesitornis unicolor), a rare overhunting, have already eliminated 1(800) BIRD YUM forest-dwelling relative of rails. The many unique Malagasy creatures. 1 (800) 247-3986 large egg, delicately colored in sal Since people first arrived on Mada mon with liver-colored spots, rested gascar 1500 to 2000 years ago, much 13330 Bessemer Street precariously in a frail, dove-like nest of the island has been deforested, Van Nuys, CA91401-3000 positioned at the end of a sloping leaving the red lateritic soil exposed (818) 997-0598 sapling. This encounter with the and eroding, with little chance for Brown Mesite is just one of many forest regeneration. -

Interspecific Social Dominance Mimicry in Birds
bs_bs_banner Zoological Journal of the Linnean Society, 2014. With 6 figures Interspecific social dominance mimicry in birds RICHARD OWEN PRUM1,2* 1Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520-8150, USA 2Peabody Natural History Museum, Yale University, New Haven, CT 06520-8150, USA Received 3 May 2014; revised 17 June 2014; accepted for publication 21 July 2014 Interspecific social dominance mimicry (ISDM) is a proposed form of social parasitism in which a subordinate species evolves to mimic and deceive a dominant ecological competitor in order to avoid attack by the dominant, model species. The evolutionary plausibility of ISDM has been established previously by the Hairy-Downy game (Prum & Samuelson). Psychophysical models of avian visual acuity support the plausibility of visual ISDM at distances ∼>2–3 m for non-raptorial birds, and ∼>20 m for raptors. Fifty phylogenetically independent examples of avian ISDM involving 60 model and 93 mimic species, subspecies, and morphs from 30 families are proposed and reviewed. Patterns of size differences, phylogeny, and coevolutionary radiation generally support the predic- tions of ISDM. Mimics average 56–58% of the body mass of the proposed model species. Mimics may achieve a large potential deceptive social advantage with <20% reduction in linear body size, which is well within the range of plausible, visual size confusion. Several, multispecies mimicry complexes are proposed (e.g. kiskadee- type flycatchers) which may coevolve through hierarchical variation in the deceptive benefits, similar to Müllerian mimicry. ISDM in birds should be tested further with phylogenetic, ecological, and experimental investigations of convergent similarity in appearance, ecological competition, and aggressive social interactions between sympatric species. -

Spiny Forest
Investigating the impact of habitat change on the bird and reptile fauna of southern Madagascar’s spiny forest Final report to African Bird Club Conservation Programme Charlie J. Gardner PhD candidate Durrell Institute of Conservation and Ecology School of Anthropology and Conservation University of Kent Canterbury Kent CT2 7NS [email protected] 29 October 2012 ALL PHOTOGRAPHS IN THIS REPORT ARE THE PROPERTY OF LOUISE JASPER AND MAY NOT BE REPRODUCED WITHOUT PERMISSION. Cover photos, clockwise from top left: 1 moderately-degraded spiny forest at Ranobe study site, 2 male Lafresnaye’s vanga (Xenopirostris xenopirostris ) perched on flowering Didierea madagascariensis , the dominant and characteristic tree of the spiny forest at Ranobe, 3 Running coua ( Coua cursor ), a spiny forest endemic and member of the endemic subfamily Couinae, 4 Adult male Furcifer labordi , the world’s only annual tetrapod. Expedition members Charlie Gardner Principal investigator Louise Jasper Research assistant (birds and reptiles) Julio-Josepha Duchene Research assistant (reptiles) Eonintsoa Research assistant (reptiles) Toto Local guide (Site 1) Alexis Local guide (Site 1) Rekamo Local guide (Site 2) Milison Justin Local guide (Site 2) Jean-Paul Local guide (Site 3) Sambiasy Local guide (Site 3) Mameno Cook (Sites 1 & 2) Saholy Cook (Site 3) Fig 1: Expedition members at Site 2, from left to right: Charlie Gardner, Eonintsoa, Julio Josepha Duchene, Louise Jasper. Front row: Milison Justin, Rekamo Background Madagascar is recognised as the world’s number one conservation priority, possessing unparalleled levels of diversity and endemism, coupled with high rates of forest loss. While the avifauna of the country is relatively impoverished with only 209 breeding species, a full 52% of these are endemic (Goodman & Hawkins 2008), giving Madagascar the highest proportion of endemic bird species of any major landmass in the world (Hawkins & Goodman 2003). -

Red Shouldered Vanga Showed Low Density at All Records Varying Between One to Three Individuals Per Hectare Per Transect Surveyed
Establishing a population -density estimate in suitable habitat range of Red-shouldered Vanga Calicalicus rufocarpalis in southwest of Madagascar By Sama Zefania 1 Calicalicus rufocarpalis Type of s piny forest Habitat 1 Po Box 185, Service of Biodiversity and environment, Cedratom, University of Toliara, Madagascar 1 Summary Red shouldered Vanga showed low density at all records varying between one to three individuals per hectare per transect surveyed. More detailed densities estimations are reported in this project allowing more accurate estimation of total population size of Red-shouldered Vanga in future. The range of Red shouldered Vanga in protected areas (Tsimanampetsotsa National Park) increase since we found new areas of presence of species in remote places of the park in which the species may take refuge. The chance to sight the species at its northern limit range (Ankilibe road to St-Augustin) starts to decrease since the human disturbance on spiny forest habitat around these sites continues to increase. The restriction of the range of this species from its north limit could continue to appear since the species became difficult to sight outside of protected areas. Further investigation is needed for determining the change on the northern and southern range limits of species. Introduction Expedition was initiated between 21 st August and 22 th December 2011 inside the band of spiny forest in which distribution of target species is known. In addition, further visits were also made before and after this above fieldwork period. Given the large patches of species range and limitation from logistic arranging and transport, we surveyed only some points which may give an idea about the home range density of species around its known distribution. -

Unlocking the Black Box of Feather Louse Diversity: a Molecular Phylogeny of the Hyper-Diverse Genus Brueelia Q ⇑ Sarah E
Molecular Phylogenetics and Evolution 94 (2016) 737–751 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Unlocking the black box of feather louse diversity: A molecular phylogeny of the hyper-diverse genus Brueelia q ⇑ Sarah E. Bush a, , Jason D. Weckstein b,1, Daniel R. Gustafsson a, Julie Allen c, Emily DiBlasi a, Scott M. Shreve c,2, Rachel Boldt c, Heather R. Skeen b,3, Kevin P. Johnson c a Department of Biology, University of Utah, 257 South 1400 East, Salt Lake City, UT 84112, USA b Field Museum of Natural History, Science and Education, Integrative Research Center, 1400 S. Lake Shore Drive, Chicago, IL 60605, USA c Illinois Natural History Survey, University of Illinois, 1816 South Oak Street, Champaign, IL 61820, USA article info abstract Article history: Songbirds host one of the largest, and most poorly understood, groups of lice: the Brueelia-complex. The Received 21 May 2015 Brueelia-complex contains nearly one-tenth of all known louse species (Phthiraptera), and the genus Revised 15 September 2015 Brueelia has over 300 species. To date, revisions have been confounded by extreme morphological Accepted 18 September 2015 variation, convergent evolution, and periodic movement of lice between unrelated hosts. Here we use Available online 9 October 2015 Bayesian inference based on mitochondrial (COI) and nuclear (EF-1a) gene fragments to analyze the phylogenetic relationships among 333 individuals within the Brueelia-complex. We show that the genus Keywords: Brueelia, as it is currently recognized, is paraphyletic. Many well-supported and morphologically unified Brueelia clades within our phylogenetic reconstruction of Brueelia were previously described as genera.