Review of Particle Size Distribution Analysis Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Guidebook to Particle Size Analysis Table of Contents
A GUIDEBOOK TO PARTICLE SIZE ANALYSIS TABLE OF CONTENTS 1 Why is particle size important? Which size to measure 3 Understanding and interpreting particle size distribution calculations Central values: mean, median, mode Distribution widths Technique dependence Laser diffraction Dynamic light scattering Image analysis 8 Particle size result interpretation: number vs. volume distributions Transforming results 10 Setting particle size specifications Distribution basis Distribution points Including a mean value X vs.Y axis Testing reproducibility Including the error Setting specifications for various analysis techniques Particle Size Analysis Techniques 15 LA-960 laser diffraction technique The importance of optical model Building a state of the art laser diffraction analyzer 18 LA-350 laser diffraction technique Compact optical bench and circulation pump in one system 19 ViewSizer 3000 nanotracking analysis A Breakthrough in nanoparticle tracking analysis 20 SZ-100 dynamic light scattering technique Calculating particle size Zeta Potential Molecular weight 25 PSA300 image analysis techniques Static image analysis Dynamic image analysis 27 Dynamic range of the HORIBA particle characterization systems 27 Selecting a particle size analyzer When to choose laser diffraction When to choose dynamic light scattering When to choose image analysis 31 References Why is particle size important? Particle size influences many properties of particulate materials and is a valuable indicator of quality and performance. This is true for powders, Particle size is critical within suspensions, emulsions, and aerosols. The size and shape of powders influences a vast number of industries. flow and compaction properties. Larger, more spherical particles will typically flow For example, it determines: more easily than smaller or high aspect ratio particles. -

Nanomaterials How to Analyze Nanomaterials Using Powder Diffraction and the Powder Diffraction File™
Nanomaterials How to analyze nanomaterials using powder diffraction and the Powder Diffraction File™ Cerium Oxide CeO2 PDF 00-064-0737 7,000 6,000 5,000 4,000 Intensity 3,000 2,000 1,000 20 30 40 50 60 70 80 90 100 110 120 Nanomaterials Table of Contents Materials with new and incredible properties are being produced around the world by controlled design at the atomic and molecular level. These nanomaterials are typically About the Powder Diffraction File ......... 1 produced in the 1-100 nm size scale, and with this small size they have tremendous About Powder Diffraction ...................... 1 surface area and corresponding relative percent levels of surface atoms. Both the size and available (reactive) surface area can contribute to unique physical properties, Analysis Tools for Nanomaterials .......... 1 such as optical transparency, high dissolution rate, and enormous strength. Crystallite Size and Particle Size ������������ 2 In this Technical Bulletin, we are primarily focused on the use of structural simulations XRPD Pattern for NaCI – An Example .... 2 in order to examine the approximate crystallite size and molecular orientation in nanomaterials. The emphasis will be on X-ray analysis of nanomaterials. However, Total Pattern Analysis and the �������������� 3 Powder Diffraction File electrons and neutrons can have similar wavelengths as X-rays, and all of the X-ray methods described have analogs with neutron and electron diffraction. The use of Pair Distribution Function Analysis ........ 3 simulations allows one to study any nanomaterials that have a known atomic and Amorphous Materials ............................ 4 molecular structure or one can use a characteristic and reproducible experimental diffraction pattern. -

I I I I Executive Summary
I I I CONTAMINANTS IN FISH AND SEDIMENTS OF THE GREAT SWAMP NATIONAL WILDLIFE REFUGE, MORRIS COUNTY, NEW JERSEY: I A 10-YEAR FOLLOW-UP INVESTIGATION DEC ID# 9950003.1 I ECDM Catalog # 5050048 I I I Prepared for: U.S. Fish & Wildlife Service Northeast Region Division of Refuges and Wildlife I Hadley, Massachusetts I Prepared by: I U.S. Fish & Wildlife Service New Jersey Field Office I Plem,antville, New Jersey I I August 2005 I Project Biologist: Clay M. Stem Assistant Supervisor: Timothy J. Kubiak I Supen,isor: Clifford G. Day I I 2 I I I I EXECUTIVE SUMMARY Located in Morris County, New Jersey about 25 miles west of New York City's Time Square, the I U.S. Fish & Wildlife Service's (Service) Great Swamp National Wildlife Refuge (GSNWR) encompasses approximately 7,500 acres ::,fwhich 3,660 acres are designated and managed as a National Wilderness Area. The GSNWR's wetlands provide important ecological functions, I including floodwater attenuation, groundwater recharge, pollution abatement, wildlife habitat, as well as recreational benefits for the public. In many portions of the Great Swamp watershed, significant areas of native soils have been disturbed by development to the extent that the I original soil profiles no longer exist. Development-related activities such as grading, infilling, and compaction have adversely altered the native soil's infiltration capacity and runoff potential I and thereby have increased storm-water 11ediment loading into many of the watershed's streams. The purpose of this investigation is to conduct a 10-year follow-up to a 1988 investigation (USFWS 1991) characterizing ambient concentrations of metals, organochlorines, and polycyclic I aromatic hydrocarbons (P AHs) in GSNWR sediments, and metals and organochlorine in fish inhabiting the GSNWR. -

A Comparative Study of Particle Size Distribution of Graphene Nanosheets Synthesized by an Ultrasound-Assisted Method
nanomaterials Article A Comparative Study of Particle Size Distribution of Graphene Nanosheets Synthesized by an Ultrasound-Assisted Method Juan Amaro-Gahete 1,† , Almudena Benítez 2,† , Rocío Otero 2, Dolores Esquivel 1 , César Jiménez-Sanchidrián 1, Julián Morales 2, Álvaro Caballero 2,* and Francisco J. Romero-Salguero 1,* 1 Departamento de Química Orgánica, Instituto Universitario de Investigación en Química Fina y Nanoquímica, Facultad de Ciencias, Universidad de Córdoba, 14071 Córdoba, Spain; [email protected] (J.A.-G.); [email protected] (D.E.); [email protected] (C.J.-S.) 2 Departamento de Química Inorgánica e Ingeniería Química, Instituto Universitario de Investigación en Química Fina y Nanoquímica, Facultad de Ciencias, Universidad de Córdoba, 14071 Córdoba, Spain; [email protected] (A.B.); [email protected] (R.O.); [email protected] (J.M.) * Correspondence: [email protected] (A.C.); [email protected] (F.J.R.-S.); Tel.: +34-957-218620 (A.C.) † These authors contributed equally to this work. Received: 24 December 2018; Accepted: 23 January 2019; Published: 26 January 2019 Abstract: Graphene-based materials are highly interesting in virtue of their excellent chemical, physical and mechanical properties that make them extremely useful as privileged materials in different industrial applications. Sonochemical methods allow the production of low-defect graphene materials, which are preferred for certain uses. Graphene nanosheets (GNS) have been prepared by exfoliation of a commercial micrographite (MG) using an ultrasound probe. Both materials were characterized by common techniques such as X-ray diffraction (XRD), Transmission Electronic Microscopy (TEM), Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). All of them revealed the formation of exfoliated graphene nanosheets with similar surface characteristics to the pristine graphite but with a decreased crystallite size and number of layers. -

Download (14Mb)
A Thesis Submitted for the Degree of PhD at the University of Warwick Permanent WRAP URL: http://wrap.warwick.ac.uk/125819 Copyright and reuse: This thesis is made available online and is protected by original copyright. Please scroll down to view the document itself. Please refer to the repository record for this item for information to help you to cite it. Our policy information is available from the repository home page. For more information, please contact the WRAP Team at: [email protected] warwick.ac.uk/lib-publications Anisotropic Colloids: from Synthesis to Transport Phenomena by Brooke W. Longbottom Thesis Submitted to the University of Warwick for the degree of Doctor of Philosophy Department of Chemistry December 2018 Contents List of Tables v List of Figures vi Acknowledgments ix Declarations x Publications List xi Abstract xii Abbreviations xiii Chapter 1 Introduction 1 1.1 Colloids: a general introduction . 1 1.2 Transport of microscopic objects – Brownian motion and beyond . 2 1.2.1 Motion by external gradient fields . 4 1.2.2 Overcoming Brownian motion: propulsion and the requirement of symmetrybreaking............................ 7 1.3 Design & synthesis of self-phoretic anisotropic colloids . 10 1.4 Methods to analyse colloid dynamics . 13 1.4.1 2D particle tracking . 14 1.4.2 Trajectory analysis . 19 1.5 Thesisoutline................................... 24 Chapter 2 Roughening up Polymer Microspheres and their Brownian Mo- tion 32 2.1 Introduction.................................... 33 2.2 Results&Discussion............................... 38 2.2.1 Fabrication and characterization of ‘rough’ microparticles . 38 i 2.2.2 Quantifying particle surface roughness by image analysis . -

Particle Size Analysis of 99Mtc-Labeled and Unlabeled Antimony Trisulfide and Rhenium Sulfide Colloids Intended for Lymphoscintigraphic Application
Particle Size Analysis of 99mTc-Labeled and Unlabeled Antimony Trisulfide and Rhenium Sulfide Colloids Intended for Lymphoscintigraphic Application Chris Tsopelas RAH Radiopharmacy, Nuclear Medicine Department, Royal Adelaide Hospital, Adelaide, Australia nature allows them to be retained by the lymph nodes (8,9) Colloidal particle size is an important characteristic to consider by a phagocytic mechanism, yet the rate of colloid transport when choosing a radiopharmaceutical for mapping sentinel through the lymphatic channels is directly related to their nodes in lymphoscintigraphy. Methods: Photon correlation particle size (10). Particles smaller than 4–5 nm have been spectroscopy (PCS) and transmission electron microscopy reported to penetrate capillary membranes and therefore (TEM) were used to determine the particle size of antimony may be unavailable to migrate through the lymphatic chan- trisulfide and rhenium sulfide colloids, and membrane filtration (MF) was used to determine the radioactive particle size distri- nel. In contrast, larger particles (ϳ500 nm) clear very bution of the corresponding 99mTc-labeled colloids. Results: slowly from the interstitial space and accumulate poorly in Antimony trisulfide was found to have a diameter of 9.3 Ϯ 3.6 the lymph nodes (11) or are trapped in the first node of a nm by TEM and 18.7 Ϯ 0.2 nm by PCS. Rhenium sulfide colloid lymphatic chain so that the extent of nodal drainage in was found to exist as an essentially trimodal sample with a contiguous areas cannot always be discerned (5). After dv(max1) of 40.3 nm, a dv(max2) of 438.6 nm, and a dv of 650–2200 injection, the radiocolloid travels to the sentinel node 99m nm, where dv is volume diameter. -

Tsi Knows Nanoparticle Measurement
TSI KNOWS NANOPARTICLE MEASUREMENT NANO INSTRUMENTATION UNDERSTANDING, ACCELERATED AEROSOL SCIENCE MEETS NANOTECHNOLOGY TSI CAN HELP YOU NAVIGATE THROUGH NANOTECHNOLOGY Our Instruments are Used by Scientists Throughout a Nanoparticle’s Life Cycle. Research and Development Health Effects–Inhalation Toxicology On-line characterization tools help researchers shorten Researchers worldwide use TSI instrumentation to generate R&D timelines. Precision nanoparticle generation challenge aerosol for subjects, quantify dose, instrumentation can produce higher quality products. and determine inhaled portion of nanoparticles. Manufacturing Process Monitoring Nanoparticle Exposure and Risk Nanoparticles are expensive. Don’t wait for costly Assess the workplace for nanoparticle emissions and off line techniques to determine if your process is locate nanoparticle sources. Select and validate engineering out of control. controls and other corrective actions to reduce worker exposure and risk. Provide adequate worker protection. 2 AEROSOL SCIENCE MEETS NANOTECHNOLOGY What Is a Nanoparticle? Types of Nanoparticles A nanoparticle is typically defined as a particle which has at Nanoparticles are made from a wide variety of materials and are least one dimension less than 100 nanometers (nm) in size. routinely used in medicine, consumer products, electronics, fuels, power systems, and as catalysts. Below are a few examples of Why Nano? nanoparticle types and applied uses: The answer is simple: better material properties. Nanomaterials have novel electrical, -

NIST Recommended Practice Guide : Particle Size Characterization
NATL INST. OF, STAND & TECH r NIST guide PUBLICATIONS AlllOb 222311 ^HHHIHHHBHHHHHHHHBHI ° Particle Size ^ Characterization Ajit Jillavenkatesa Stanley J. Dapkunas Lin-Sien H. Lum Nisr National Institute of Specidl Standards and Technology Publication Technology Administration U.S. Department of Commerce 960 - 1 NIST Recommended Practice Gu Special Publication 960-1 Particle Size Characterization Ajit Jillavenkatesa Stanley J. Dapkunas Lin-Sien H. Lum Materials Science and Engineering Laboratory January 2001 U.S. Department of Commerce Donald L. Evans, Secretary Technology Administration Karen H. Brown, Acting Under Secretary of Commerce for Technology National Institute of Standards and Technology Karen H. Brown, Acting Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publication 960-1 Natl. Inst. Stand. Technol. Spec. Publ. 960-1 164 pages (January 2001) CODEN: NSPUE2 U.S. GOVERNMENT PRINTING OFFICE WASHINGTON: 2001 For sale by the Superintendent of Documents U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: (202)512-1800 Fax: (202)512-2250 Mail: Stop SSOP, Washington, DC 20402-0001 Preface PREFACE Determination of particle size distribution of powders is a critical step in almost all ceramic processing techniques. The consequences of improper size analyses are reflected in poor product quality, high rejection rates and economic losses. -

Introduction to Dynamic Light Scattering for Particle Size Determination
www.horiba.com/us/particle Jeffrey Bodycomb, Ph.D. Introduction to Dynamic Light Scattering for Particle Size Determination © 2016 HORIBA, Ltd. All rights reserved. 1 Sizing Techniques 0.001 0.01 0.1 1 10 100 1000 Nano-Metric Fine Coarse SizeSize Colloidal Macromolecules Powders AppsApps Suspensions and Slurries Electron Microscope Acoustic Spectroscopy Sieves Light Obscuration Laser Diffraction – LA-960 Electrozone Sensing Methods DLS – SZ-100 Sedimentation Disc-Centrifuge Image Analysis PSA300, Camsizer © 2016 HORIBA, Ltd. All rights reserved. 3 Two Approaches to Image Analysis Dynamic: Static: particles flow past camera particles fixed on slide, stage moves slide 0.5 – 1000 um 1 – 3000 um 2000 um w/1.25 objective © 2016 HORIBA, Ltd. All rights reserved. 4 Laser Diffraction Laser Diffraction •Particle size 0.01 – 3000 µm •Converts scattered light to particle size distribution •Quick, repeatable •Most common technique •Suspensions & powders © 2016 HORIBA, Ltd. All rights reserved. 5 Laser Diffraction Suspension Powders Silica ~ 30 nm Coffee Results 0.3 – 1 mm 14 100 90 12 80 10 70 60 8 50 q(%) 6 40 4 30 20 2 10 0 0 10.00 100.0 1000 3000 Diameter(µm) © 2016 HORIBA, Ltd. All rights reserved. 6 What is Dynamic Light Scattering? • Dynamic light scattering refers to measurement and interpretation of light scattering data on a microsecond time scale. • Dynamic light scattering can be used to determine – Particle/molecular size – Size distribution – Relaxations in complex fluids © 2016 HORIBA, Ltd. All rights reserved. 7 Other Light Scattering Techniques • Static Light Scattering: over a duration of ~1 second. Used for determining particle size (diameters greater than 10 nm), polymer molecular weight, 2nd virial coefficient, Rg. -
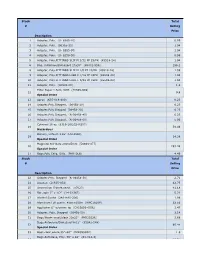
Stock Total # Selling Price 1 Adapter, Poly. (U- 6365-10)
Stock Total # Selling Price Description 1 Adapter, Poly. (U- 6365-10) 0.99 2 Adapter, Poly. (06365-22) 1.04 3 Adapter, Poly. (U- 6365-30) 1.04 4 Adapter, Poly. (U- 6359-50) 0.99 5 Adapter, Poly,FITTINGS SLIP M 3/32 PP 25/PK (45518-24) 1.04 6 Bag, Autoclave/Biohazard 24x30'' (95042-556) 156.1 8 Adapter, Poly.FITTINGS SLIP M 1/8 PP 25/PK (45518-26) 1.59 9 Adapter, Poly.FITTINGS LUER F 1/16 PP 25PK (45508-00) 1.04 10 Adapter, Poly.FITTINGS LUER F 3/32 PP 25PK (45508-02) 1.04 11 Adapter, Poly. (30800-08) 1.3 Filter Paper 1.5cm, VWR (28309-989) 12 9.9 Special Order 13 Apron (635-018-400) 0.23 14 Adapter,Poly.Stepped, (06458-10) 6.23 15 Adapter,Poly.Stepped (06458-20) 6.23 16 Adapter,Poly.Stepped, (N-06458-40) 6.23 17 Adapter,Poly.Stepped, (N-06458-60) 5.06 Cytoseal 16 oz (8310-16/23244257) 18 58.49 Hazardous Battery, Lithium 3.6V (LS14500) 19 14.38 Special Order Magnetic Stir Bars 2mmx5mm (58948-377) 20 293.48 Special Order 21 Bags,Poly Cello,10 lb. (PKR10Lb) 4.49 Stock Total # Selling Price Description 22 Adapter,Poly. Stepped (N-06458-30) 2.71 24 Alconox (21835-032) 42.75 25 Ammonium Sulfate,certif. (A7023) 41.18 26 Bar, spin 2'' x 1/2'' (14-51367) 5.22 27 Alcohol Swabs (248-HAS-200) 1.96 28 Aluminum Foil comm. 45cmx100m (WPC1810P) 32.56 29 Applicator 6'' w/cotton tip (CA10806-000L) 1.47 30 Adapter, Poly. -

Digestion of Algin by Pseudomonas Maltophilia and Pseudomonas Putida V
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Jan. 1980, p. 92-96 Vol. 39, No. 1 0099-2240/80/01-0092/05$02.00/0 Digestion of Algin by Pseudomonas maltophilia and Pseudomonas putida V. LYLE VON RIESEN Department ofMedical Microbiology, College ofMedicine, University of Nebraska Medical Center, Omaha, Nebraska 68105 Pseudomonas maltophilia and Pseudomonas putida were identified as al- ginolytic species. Two media used for demonstrating alginolytic activity are described. The applied aspects of the ability of these two species to digest algin are discussed. In a search for undescribed biochemical activ- Both media contained yeast extract (0.5%), algin (al- ities of nonfastidious, nonfermentative gram- ginic acid, sodium salt, no. A-7128; Sigma Chemical negative bacilli (NFB) which might be used to Co., St. Louis, Mo.) (1%), and bromothymol blue of these taxonomically (0.002%). Agar (0.5%) was used to form an algin-agar aid in the identification medium and animal charcoal (0.1%) to form an algin- troublesome organisms, a survey of a variety of charcoal medium. The media were prepared in 100- to complex polysaccharides as substrates was made 500-ml amounts in the following manner. Calculated by using a few strains of some well-recognized amounts of yeast extract and bromothymol blue (from species of NFB. When it was discovered that a 1% aqueous solution) were added to an appropriate some of the strains ofPseudomonas maltophilia volume of distilled water. This was then warmed on a showed evidence of an ability to hydrolyze algin magnetic stirrer-heater. When warm (40 to 60°C), (sodium alginate), an available collection of ap- algin and charcoal (algin-charcoal medium) or algin proximately 390 strains of NFB was examined and agar (algin-agar medium) were sprinkled into the of individual strains to digest vigorously stirred solution. -

Maggie Williams Particle Size, Shape and Sorting: What Grains Can Tell Us
Particle size, shape and sorting: what grains can tell us Maggie Williams Most sediments contain particles that have a range of sizes, so the mean or average grain size is used in description. Mean grain size of loose sediments is measured by size analysis using sieves Grain size >2mm Coarse grain size (Granules<pebble<cobbles<boulders) 0.06 to 2mm Medium grain size Sand: very coarse-coarse-medium-fine-very fine) <0.06mm Fine grain size (Clay<silt) Difficult to see Remember that for sediment sizes > fine sand, the coarser the material the greater the flow velocity needed to erode, transport & deposit the grains Are the grains the same size of different? What does this tell you? If grains are the same size this tells you that the sediment was sorted out during longer transportation (perhaps moved a long distance by a river or for a long time by the sea. If grains are of different sizes the sediment was probably deposited close to its source or deposited quickly (e.g. by a flood or from meltwater). Depositional environments Environment 1 Environment 2 Environment 3 Environment 4 Environment 5 Environment 6 Sediment size frequency plots from different depositional environments • When loose sediment collected from a sedimentary environment is washed and then sieved it is possible to measure the grain sizes in the sediment accurately. • The grain size distribution may then be plotted as a histogram or as a cumulative frequency curve. • Sediments from different depositional environments give different sediment size frequency plots. This shows the grain size distribution for a river sand.