Modelling and Control of the Human Cardiovascular System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Market Analysis of Clinical Cardiology 2020
Interventional Cardiology Journal 2019 Market Analysis ISSN 2471-8157 Vol.5 No.3 Market Analysis of Clinical Cardiology 2020 Emily Professor of Epidemiology and Public Health at Australian National University Australian Capital Territory, Australia, E-mail: [email protected] Hilaris Conferences heartily invites you to the Clinical Target Audience Cardiology in San Fransisco, USA during October 26-27, 2020, • Directors of Hypertension or related Programs or to uncover your research ideas and work on heart heath, Associations cardiac diagnosis, and cardiac surgery comes under Clinical • Heads, Deans and Professors of Hypertension or Cardiology Cardiology research. Cardiology is a medical specialty and a departments branch of internal medicine concerned with treatment of • Scientists and Researchers organizers disorders of the heart and the blood vessels. Hilaris • Doctors Conferences is assuring Clinical Cardiology 2020 will provide • Medical Colleges you that international platform to explore your recent paper in • Writers front of renowned keynote speakers, delegates, CEOs, • Healthcare professionals Professors and Doctors. We are waiting to have you in San • Founders and Employees of the related companies Fransisco. • Clinical investigators • Hospitals and Health Services Market Analysis • Pharmaceutical companies • Training institutions Cardiovascular disease is the major cause of death across • Support organizers the globe. It estimated that for 17.3 million deaths in 2015 and • Data Management Companies is further anticipated to claim 23.6 million lives in 2030 • Cardiologists training and education (according to estimation by the World Health Organization). • Nurse and nursing education institutions The International Diabetes Federation has estimating that approximately 415 million people were diabetic in 2015 while the number is expected to increase 642 million worldwide by Related Companies/Industries 2040. -

The Ottawa Cardiovascular Centre Is Pleased Concept and Capacity
502-1355 Bank Street, Ottawa, ON K1H 8K7 T 613-738-1584 F 613-738-9097 E [email protected] The Ottawa Cardiovascular Centre is pleased to announce the expansion of our clinic in terms of concept and capacity RAPID ACCESS • SHORT WAIT LISTS • EXPANDED SERVICES We have hired two Physician Assistants to extend and enhance our services. We have added a second pediatric cardiologist. In addition, we now offer: EXPANDED NON-INVASIVE SERVICES: Nuclear Cardiology Imaging expanded with 2 state of the art CZT solid state cameras Echocardiography expanded with addition for rapid acquisition/lower radiation of 4 state of the art GE Vivid E 90 systems • Treadmill or persantine stress myocardial • No wait times perfusion imaging • Echo contrast enhances difficult imaging RAPID REFERRAL CLINIC: • Treadmill and bicycle stress Atrial fibrillation/anticoagulation, chest pain, echocardiography palpitation/syncope, shortness of breath, • Adult and pediatric echocardiography post ER visit Arrhythmia Detection: Real time wireless RESIDUAL RISK CLINIC: prospective monitoring for immediate Optimization of diabetes, dyslipidemia, arrhythmia detection and notification hypertension, post revascularization, • 2 day holter monitoring vascular risk reduction, LV function/HF • 3 day holter: retrospective quantitative CardioOncology, adult congenital HD, analysis (on site hook-up and mail out first responders/sports – ischaemic Mini Holter) risk assessment Please note that e-Referral is now available via the OCEAN e-Referral Healthmap 502-1355 Bank Street, Ottawa, -

TRPA1 Is Essential for the Vascular Response to Environmental Cold Exposure
ARTICLE Received 18 Aug 2014 | Accepted 3 Nov 2014 | Published 11 Dec 2014 DOI: 10.1038/ncomms6732 OPEN TRPA1 is essential for the vascular response to environmental cold exposure Aisah A. Aubdool1, Rabea Graepel1, Xenia Kodji1, Khadija M. Alawi1, Jennifer V. Bodkin1, Salil Srivastava1, Clive Gentry2, Richard Heads1, Andrew D. Grant2, Elizabeth S. Fernandes1, Stuart Bevan2 & Susan D. Brain1 The cold-induced vascular response, consisting of vasoconstriction followed by vaso- dilatation, is critical for protecting the cutaneous tissues against cold injury. Whilst this physiological reflex response is historic knowledge, the mechanisms involved are unclear. Here by using a murine model of local environmental cold exposure, we show that TRPA1 acts as a primary vascular cold sensor, as determined through TRPA1 pharmacological antagonism or gene deletion. The initial cold-induced vasoconstriction is mediated via TRPA1-dependent superoxide production that stimulates a2C-adrenoceptors and Rho-kinase-mediated MLC phosphorylation, downstream of TRPA1 activation. The subsequent restorative blood flow component is also dependent on TRPA1 activation being mediated by sensory nerve-derived dilator neuropeptides CGRP and substance P, and also nNOS-derived NO. The results allow a new understanding of the importance of TRPA1 in cold exposure and provide impetus for further research into developing therapeutic agents aimed at the local protection of the skin in disease and adverse climates. 1 BHF Cardiovascular Centre of Excellence and Centre of Integrative Biomedicine, Cardiovascular Division, King’s College London, London SE1 9NH, UK. 2 Wolfson Centre for Age Related Diseases, King’s College London, London SE1 1UL, UK. Correspondence and requests for materials should be addressed to S.D.B. -

Cardiovascular Disease Prevention and Control Translating Evidence
Cardiovascular disease prevention and control Translating evidence into action 3 WHO Library Cataloguing-in-Publication Data Cardiovascular disease prevention and control: translating evidence into action. 1.Cardiovascular diseases - prevention and control 2.Cerebrovascular accident - prevention and control 3.Rheumatic heart disease - prevention and control 4.Risk reduction behavior I.World Health Organization. ISBN 92 4 159325 3 (NLM classification: WG 120) © World Health Organization 2005 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for per- mission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatso- ever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Endogenously Released Adenosine Causes Pulmonary Vasodilation During the Acute Phase of Pulmonary Embolization in Dogs☆
IJC Heart & Vasculature 24 (2019) 100396 Contents lists available at ScienceDirect IJC Heart & Vasculature journal homepage: http://www.journals.elsevier.com/ijc-heart-and-vasculature Endogenously released adenosine causes pulmonary vasodilation during the acute phase of pulmonary embolization in dogs☆ Hiroko Takahama a,b,c, Hiroshi Asanuma d, Osamu Tsukamoto e,ShinItoa, Masafumi Kitakaze a,⁎ a Department of Clinical Research and Development, National Cerebral and Cardiovascular Center, 5-7-1 Fujishirodai, Suita, Osaka, Japan b Department of Cell Biology, National Cerebral and Cardiovascular Center, 5-7-1 Fujishirodai, Suita, Osaka, Japan c Department of Molecular Imaging in Cardiovascular Medicine, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka, Japan d Department of Internal Medicine, Meiji University of Integrative Medicine, Hiyoshicho, Nantan, Kyoto, Japan e Department of Medical Biochemistry, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka, Japan article info abstract Article history: Background: Endogenous adenosine levels increase under stress in various organs. Exogenously administered Received 31 March 2019 adenosine is a well-known pulmonary vasodilator. However, the physiology and therapeutic potential of endog- Received in revised form 25 May 2019 enous adenosine during alteration in pulmonary hemodynamics such as pulmonary embolism is not elucidated. Accepted 24 June 2019 We hypothesized that the adenosine level increases following an acute elevation of pulmonary resistance, Available online 10 July 2019 resulting in pulmonary vasodilation. Methods: We induced acute pulmonary embolization by injecting plastic beads in anesthetized dogs. Plasma Keywords: adenosine levels, defined as the product of plasma adenosine concentration and simultaneous cardiac output, Adenosine Pulmonary embolization were assessed from blood samples from the superior vena cava, main pulmonary artery (MPA), and ascending Hemodynamics aorta 1 and 10 min following injection. -

Regulation of Blood Pressure
REGULATION OF BLOOD PRESSURE Prof. Sultan Ayoub Meo MBBS, Ph.D (Pak), M Med Ed (Dundee), FRCP (London), FRCP (Dublin), FRCP (Glasgow), FRCP (Edinburgh) Professor and Consultant, Department of Physiology, College of Medicine, King Saud University, Riyadh, KSA LECTURE OBJECTIVES ▪ Factors regulating arterial blood pressure ▪ Explain how they influence arterial blood pressure. ▪ Physiological importance of regulating arterial blood pressure ▪ Discuss short term, intermediate and long-term regulation of blood pressure; nervous, hormonal and renal regulation of arterial blood pressure. REGULATION OF BLOOD PRESSURE REGULATION OF BLOOD PRESSURE SHORT TERM REGULATION OF BLOOD PRESSURE Rapidly acting within seconds to minutes 1. Baroreceptors Reflex Mechanism 2. Chemoreceptors Mechanism 3. CNS Ischemic Response Mechanism 4. Atrial Stretch Volume Receptors 1. THE BARORECEPTORS Changes in MAP are detected by baroreceptors (pressure receptors) in the carotid and aortic arteries. Carotid baroreceptors are located in the carotid sinus, both sides of the neck. Aortic baroreceptors are located in the aortic arch. These receptors provide information to the cardiovascular centres in the medulla oblongata about the degree of stretch with pressure changes. 1. THE BARORECEPTORS Guyton and Hall, pp 174 1. THE BARORECEPTORS ❑ At normal arterial pressure the baroreceptors are active. ❑ Increased blood pressure increases their rate of activity, while decreased pressure decreases the rate of firing (activity). ❑ They play an important role in maintaining relatively constant blood flow to vital organs such as brain during rapid changes in pressure such as standing up after lying down. That is why they are called “pressure buffers”. 1. THE BARORECEPTORS 1. THE BARORECEPTORS ↑ MAP Baroreceptors ↑ Parasympathetic ↓ Sympathetic (vagal) activity activity ↓ HR ↓ SV ↓ TPR ↓ MAP 1. -

Characteristics, Aetiological Spectrum and Management of Valvular Heart
+Model ACVD-954; No. of Pages 8 ARTICLE IN PRESS Archives of Cardiovascular Disease (2017) xxx, xxx—xxx Available online at ScienceDirect www.sciencedirect.com CLINICAL RESEARCH Characteristics, aetiological spectrum and management of valvular heart disease in a Tunisian cardiovascular centre Les particularités, le profil étiologique et la prise en charge des valvulopathies acquises dans un centre cardiovasculaire en Tunisie a,∗ b a Faten Triki , Jihen Jdidi , Dorra Abid , a a Nada Tabbabi , Selma Charfeddine , a a a Sahar Ben Kahla , Mourad Hentati , Leila Abid , a Samir Kammoun a Department of Cardiology, Hédi Chaker Hospital, University of Sfax, El Ain Street Km 0.5, 3029 Sfax, Tunisia b Department of Epidemiology, Hédi Chaker Hospital, University of Sfax, Sfax, Tunisia Received 21 March 2016; accepted 1st August 2016 KEYWORDS Summary Valvular heart Background. — Valvular heart diseases occur frequently in Tunisia, but no precise statistics are disease; available. Cardiac surgery; Aim. — To analyse the characteristics of patients with abnormal valvular structure and function, Heart valve; and to identify the aetiological spectrum, treatment and outcomes of valvular heart disease in Rheumatic heart a single cardiovascular centre in Tunisia. disease Methods. — This retrospective study included patients with abnormal valvular structure and function, who were screened by transthoracic echocardiography at a single cardiology depart- ment between January 2010 and December 2013. Data on baseline characteristics, potential aetiology, treatment strategies and discharge outcomes were collected from medical records. Results. — There were 959 patients with a significant valvular heart disease (mean age 53 ± 17 years; female/male ratio 0.57). Valvular heart disease was native in 77% of patients. -
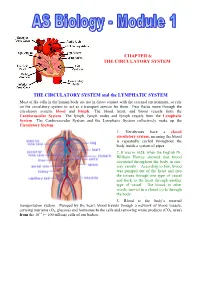
The Circulatory System
CHAPTER 6: THE CIRCULATORY SYSTEM THE CIRCULATORY SYSTEM and the LYMPHATIC SYSTEM Most of the cells in the human body are not in direct contact with the external environment, so rely on the circulatory system to act as a transport service for them. Two fluids move through the circulatory system: blood and lymph. The blood, heart, and blood vessels form the Cardiovascular System. The lymph, lymph nodes and lymph vessels form the Lymphatic System. The Cardiovascular System and the Lymphatic System collectively make up the Circulatory System. 1. Vertebrates have a closed circulatory system, meaning the blood is repeatedly cycled throughout the body inside a system of pipes. 2. It was in 1628, when the English Dr. William Harvey showed that blood circulated throughout the body in one- way vessels. According to him, blood was pumped out of the heart and into the tissues through one type of vessel and back to the heart through another type of vessel. The blood, in other words, moved in a closed cycle through the body. 3. Blood is the body’s internal transportation system. Pumped by the heart, blood travels through a network of blood vessels, carrying nutrients (O2, glucose) and hormones to the cells and removing waste products (CO2. urea) from the 1012 (= 100 trillion) cells of our bodies.. THE HEART 1. The central organ of the cardiovascular system is the heart. This is a hollow, muscular organ that contracts at regular intervals, forcing blood through the circulatory system. 2. The heart is cone-shaped, about the size of a fist, and is located in the centre of the thorax, between the lungs, directly behind the sternum (breastbone). -

Sleep Apnoea and Systemic Hypertension
Thorax: first published as 10.1136/thx.44.12.984 on 1 December 1989. Downloaded from Thorax 1989;44:984-989 Review Sleep apnoea and systemic hypertension Gross obstructive sleep apnoea has two clear pitfalls and problems with these experimental and consequences, recurrent sleep disruption (with epidemiological data, which call for careful scrutiny daytime sleepiness) and repeated noctural hypox- before health screening programmes start to label aemia. The importance of the hypoxaemia is debated snoring as more than an auditory nuisance. but when it is severe, or perhaps in the presence of This review divides the lines of argument and ischaemic heart disease, it may trigger off serious evidence into five parts: (1) How does obstructive sleep arrhythmias.' When associated with even quite mild apnoea raise systemic blood pressure acutely? (2) Does lower airways obstruction, obstructive sleep apnoea obstructive sleep apnoea really raise diurnal systemic may lead to diurnal hypoxaemia and hypercapnia23 blood pressure in the long term and increase cardio- with all the well known sequelae of fluid retention, vascular complications? (3) What is the evidence that pulmonary hypertension, and right ventricular hyper- an important proportion of patients with essential trophy (cor pulmonale).4 Why profound intermittent hypertension have appreciable sleep apnoea? (4) What hypoxaemia may apparently be harmless is not clear, is the epidemiological evidence that snoring is an but the key may be in its short duration and the independent risk factor for systemic hypertension and recovery of arterial oxygen tension (Pao2) between cardiovascular events? (5) What evidence do we need episodes ofapnoea. For example, this pulsatile hypox- to establish whether snoring and sleep apnoea are aemia does not lead to raised erythropoietin concen- important and underdiagnosed causes of essential trations,5 which explains the rarity ofpolycythaemia in hypertension or cardiovascular events? copyright. -

Challenging a Proposed Role for TRPC5 in Aortic Baroreceptor Pressure-Sensing
DOI: 10.1038/s41467-017-02703-w OPEN Correspondence: Challenging a proposed role for TRPC5 in aortic baroreceptor pressure-sensing Pratish Thakore1,2, Susan D. Brain1 & David J. Beech3 Nature Communications 9: 1245 doi:10.1038/s41467-017-02703-w (2018) Lau et al.1 have suggested a role for TRPC5 in the control of We have corresponded with Professor Xiaoqiang Yao of Lau et al.1 blood pressure where they suggest TRPC5 has a pivotal role in to try to resolve these discrepancies. Professor Yao has informed us, baroreflex regulation. after re-calculation of his data, that there was an analytical error. 1234567890():,; As part of this work it was specified that TRPC5 Correcting for this mistake, their results now also show no change in knockout (KO) mice gifted from Professor David Clapham’s baseline blood pressure between WT and TRPC5 KO mice. laboratory have elevated mean arterial pressure; WT (wildtype) Although disturbance of a mechanical force-sensing 113 ± 7 mmHg vs. KO 158 ± 26 mmHg (n = 12, p < 0.05, Fig. 8 mechanism in baroreceptors might not necessarily affect blood in the original paper1). In addition, the representative traces pressure, Lau et al.1 reported an effect that they defined as severe from one WT and one KO mouse showed no change in diurnal and suggested that it was explained by a TRPC5 mechanical variation in blood pressure or heart rate, which is unusual (Fig. 8 force-sensing mechanism in the baroreceptors. On the basis of in ref. 1). Furthermore, there was no indication of what the understanding that they have now changed their position on time periods correlated to the light and dark phases. -

A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes from the Adult Mouse Heart
New Methods in Cardiovascular Biology A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart Matthew Ackers-Johnson, Peter Yiqing Li, Andrew P. Holmes, Sian-Marie O’Brien, Davor Pavlovic, Roger S. Foo Rationale: Cardiovascular disease represents a global pandemic. The advent of and recent advances in mouse genomics, epigenomics, and transgenics offer ever-greater potential for powerful avenues of research. However, progress is often constrained by unique complexities associated with the isolation of viable myocytes from the adult mouse heart. Current protocols rely on retrograde aortic perfusion using specialized Langendorff apparatus, which poses considerable logistical and technical barriers to researchers and demands extensive training investment. Objective: To identify and optimize a convenient, alternative approach, allowing the robust isolation and culture of Downloaded from adult mouse cardiac myocytes using only common surgical and laboratory equipment. Methods and Results: Cardiac myocytes were isolated with yields comparable to those in published Langendorff- based methods, using direct needle perfusion of the LV ex vivo and without requirement for heparin injection. Isolated myocytes can be cultured antibiotic free, with retained organized contractile and mitochondrial morphology, transcriptional signatures, calcium handling, responses to hypoxia, neurohormonal stimulation, and electric pacing, http://circres.ahajournals.org/ and are amenable to patch clamp and adenoviral gene transfer techniques. Furthermore, the methodology permits concurrent isolation, separation, and coculture of myocyte and nonmyocyte cardiac populations. Conclusions: We present a novel, simplified method, demonstrating concomitant isolation of viable cardiac myocytes and nonmyocytes from the same adult mouse heart. We anticipate that this new approach will expand and accelerate innovative research in the field of cardiac biology. -

Feasibility and Clinical Decision-Making with 3D Echocardiography in Routine Practice J L Hare,1 C Jenkins,1 S Nakatani,2 a Ogawa,2 C-M Yu,3 T H Marwick4
Downloaded from heart.bmj.com on September 6, 2014 - Published by group.bmj.com Cardiac imaging and non-invasive imaging Feasibility and clinical decision-making with 3D echocardiography in routine practice J L Hare,1 C Jenkins,1 S Nakatani,2 A Ogawa,2 C-M Yu,3 T H Marwick4 1 University of Queensland, ABSTRACT more accurate for assessing LV volumes and EF, 2 Brisbane, Australia; National Objective: To assess the feasibility and potential impact with less test-retest variability and better inter- Cardiovascular Centre, Osaka, observer reproducibility than 2DE.4 These observa- Japan; 3 The Chinese University of routine three-dimensional (3D) echocardiographic of Hong Kong, Hong Kong, assessment of left ventricular (LV) ejection fraction and tions have extended to people with both normal People’s Republic of China; volumes on clinical decision-making. and abnormal LV morphology,6 with an accuracy 4 University of Queensland, Methods: Patients referred to three hospital-based comparable to cardiac MRI and exceeding that of Brisbane, Australia echocardiography laboratories underwent 2D echocardio- nuclear techniques.7 Despite these findings, the Correspondence to: graphy (2DE) and 3D echocardiography (3DE). Feasibility clinical feasibility of RT-3DE in routine practice Professor T H Marwick, was assessed in a group of 168 unselected patients and and its incremental benefit over 2DE in terms of University of Queensland decision-making assessed within an expanded group of clinical decision-making are undefined.89 In this Department of Medicine, study, we sought to evaluate the feasibility and Princess Alexandra Hospital, 220 patients. The time for acquisition and measurement Ipswich Road, Brisbane, Q4102, was obtained.