MINERALOGICAL NOTES THERMAL STABILITY of AZURITE and MALACHITE Der,B R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rediscovery of the Elements — a Historical Sketch of the Discoveries
REDISCOVERY OF THE ELEMENTS — A HISTORICAL SKETCH OF THE DISCOVERIES TABLE OF CONTENTS incantations. The ancient Greeks were the first to Introduction ........................1 address the question of what these principles 1. The Ancients .....................3 might be. Water was the obvious basic 2. The Alchemists ...................9 essence, and Aristotle expanded the Greek 3. The Miners ......................14 philosophy to encompass a obscure mixture of 4. Lavoisier and Phlogiston ...........23 four elements — fire, earth, water, and air — 5. Halogens from Salts ...............30 as being responsible for the makeup of all 6. Humphry Davy and the Voltaic Pile ..35 materials of the earth. As late as 1777, scien- 7. Using Davy's Metals ..............41 tific texts embraced these four elements, even 8. Platinum and the Noble Metals ......46 though a over-whelming body of evidence 9. The Periodic Table ................52 pointed out many contradictions. It was taking 10. The Bunsen Burner Shows its Colors 57 thousands of years for mankind to evolve his 11. The Rare Earths .................61 thinking from Principles — which were 12. The Inert Gases .................68 ethereal notions describing the perceptions of 13. The Radioactive Elements .........73 this material world — to Elements — real, 14. Moseley and Atomic Numbers .....81 concrete basic stuff of this universe. 15. The Artificial Elements ...........85 The alchemists, who devoted untold Epilogue ..........................94 grueling hours to transmute metals into gold, Figs. 1-3. Mendeleev's Periodic Tables 95-97 believed that in addition to the four Aristo- Fig. 4. Brauner's 1902 Periodic Table ...98 telian elements, two principles gave rise to all Fig. 5. Periodic Table, 1925 ...........99 natural substances: mercury and sulfur. -

Mottramite Pbcu(VO4)(OH) C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic
Mottramite PbCu(VO4)(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m 2/m 2/m. As crystals, equant or dipyramidal {111}, prismatic [001] or [100], with {101}, {201}, many others, to 3 mm, in drusy crusts, botryoidal, usually granular to compact, massive. Physical Properties: Fracture: Small conchoidal to uneven. Tenacity: Brittle. Hardness = 3–3.5 D(meas.) = ∼5.9 D(calc.) = 6.187 Optical Properties: Transparent to nearly opaque. Color: Grass-green, olive-green, yellow- green, siskin-green, blackish brown, nearly black. Streak: Yellowish green. Luster: Greasy. Optical Class: Biaxial (–), rarely biaxial (+). Pleochroism: Weak to strong; X = Y = canary-yellow to greenish yellow; Z = brownish yellow. Orientation: X = c; Y = b; Z = a. Dispersion: r> v,strong; rarely r< v.α= 2.17(2) β = 2.26(2) γ = 2.32(2) 2V(meas.) = ∼73◦ Cell Data: Space Group: P nma. a = 7.667–7.730 b = 6.034–6.067 c = 9.278–9.332 Z=4 X-ray Powder Pattern: Mottram St. Andrew, England; close to descloizite. 3.24 (vvs), 5.07 (vs), 2.87 (vs), 2.68 (vs), 2.66 (vs), 2.59 (vs), 1.648 (vs) Chemistry: (1) (2) (1) (2) CrO3 0.50 ZnO 0.31 10.08 P2O5 0.24 PbO 55.64 55.30 As2O5 1.33 H2O 3.57 2.23 V2O5 21.21 22.53 insol. 0.17 CuO 17.05 9.86 Total 100.02 100.00 (1) Bisbee, Arizona, USA; average of three analyses. (2) Pb(Cu, Zn)(VO4)(OH) with Zn:Cu = 1:1. -

Crystals of an Insoluble Carbonate of Copper Grown Under a Soda Solution L
Journal of the Arkansas Academy of Science Volume 1 Article 22 1941 Crystals of an Insoluble Carbonate of Copper Grown under a Soda Solution L. B. Roberts University of Arkansas at Monticello Edwin Roberts University of Arkansas at Monticello Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Organic Chemistry Commons Recommended Citation Roberts, L. B. and Roberts, Edwin (1941) "Crystals of an Insoluble Carbonate of Copper Grown under a Soda Solution," Journal of the Arkansas Academy of Science: Vol. 1 , Article 22. Available at: http://scholarworks.uark.edu/jaas/vol1/iss1/22 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 1 [1941], Art. 22 CRYSTALS OF AN INSOLUBLE CARBONATE OF COPPER GROWN UNDER A SODA SOLUTION1 L. B. Roberts and Edwin Roberts, Arkansas Agricultural and Mechanical College, Monticello When an old fire extinguisher of the soda-acid type was opened and emptied preparatory to recharging, several grams of blue crystals were found in the bottom of the container. -

Formation of Chrysocolla and Secondary Copper Phosphates in the Highly Weathered Supergene Zones of Some Australian Deposits
Records of the Australian Museum (2001) Vol. 53: 49–56. ISSN 0067-1975 Formation of Chrysocolla and Secondary Copper Phosphates in the Highly Weathered Supergene Zones of Some Australian Deposits MARTIN J. CRANE, JAMES L. SHARPE AND PETER A. WILLIAMS School of Science, University of Western Sydney, Locked Bag 1797, Penrith South DC NSW 1797, Australia [email protected] (corresponding author) ABSTRACT. Intense weathering of copper orebodies in New South Wales and Queensland, Australia has produced an unusual suite of secondary copper minerals comprising chrysocolla, azurite, malachite and the phosphates libethenite and pseudomalachite. The phosphates persist in outcrop and show a marked zoning with libethenite confined to near-surface areas. Abundant chrysocolla is also found in these environments, but never replaces the two secondary phosphates or azurite. This leads to unusual assemblages of secondary copper minerals, that can, however, be explained by equilibrium models. Data from the literature are used to develop a comprehensive geochemical model that describes for the first time the origin and geochemical setting of this style of economically important mineralization. CRANE, MARTIN J., JAMES L. SHARPE & PETER A. WILLIAMS, 2001. Formation of chrysocolla and secondary copper phosphates in the highly weathered supergene zones of some Australian deposits. Records of the Australian Museum 53(1): 49–56. Recent exploitation of oxide copper resources in Australia these deposits are characterized by an abundance of the has enabled us to examine supergene mineral distributions secondary copper phosphates libethenite and pseudo- in several orebodies that have been subjected to intense malachite associated with smaller amounts of cornetite and weathering. -

Reduction Spheroids from the Upper Carboniferous Hopewell Group, Dorchester Cape, New Brunswick: Notes on Geochemistry, Mineralogy and Genesis
Atlantic Geology 159 Reduction spheroids from the Upper Carboniferous Hopewell Group, Dorchester Cape, New Brunswick: notes on geochemistry, mineralogy and genesis Allan W. Lines 1, John Parnell^, and David J. Mossman^ * 1 Department of Physics, Engineering and Geoscience, Mount Allison University, Sackville, New Brunswick EOA 3C0, Canada 2 School of Geosciences, The Queen’s University of Belfast, Belfast, BT71NM, United Kingdom Date Received September 29, 1995 Date Accepted December 18, 1995 The bajada-playa sequence of terrestrial Upper Carboniferous redbeds of the Hopewell Group at Dorchester Cape, New Brunswick, hosts innumerable reduction spheroids in fine- to coarse-grained clastic sedimentary rocks, paleosols and caliche beds. The spheroids are grey-green, typically 2 to 3 cm in diameter, and may contain a dark, mineralized central core and less commonly one or more mineralized rings, concentric about the core. They de crease progressively in average diameter from 2.75 cm at the base to 0.85 cm at the top of an overall upward- fining 250 m thick measured stratigraphic section. Conditions controlling the genesis of the spheroids were established shortly after sedimentation. Develop ment was subtly controlled by groundwater flow patterns and by various sedimentary structures. The enclosing redbeds provide an adequate source for the metals contained in the mineralized spheroids. Low-temperature chlo ride complexes originating from evaporative fluids concentrated during redbed formation are believed to have been responsible for transport of the metals to reduction sites. Precipitation probably occurred as a result of a change in redox potential governed by electrical self-potentials of various detrital and early diagenetic grains, particularly pyrite and/or Fe-Ti oxides. -

Geology and Mineralogy of the Ape.X Washington County, Utah
Geology and Mineralogy of the Ape.x Germanium-Gallium Mine, Washington County, Utah Geology and Mineralogy of the Apex Germanium-Gallium Mine, Washington County, Utah By LAWRENCE R. BERNSTEIN U.S. GEOLOGICAL SURVEY BULLETIN 1577 DEPARTMENT OF THE INTERIOR DONALD PAUL HODEL, Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON: 1986 For sale by the Distribution Branch, Text Products Section U.S. Geological Survey 604 South Pickett St. Alexandria, VA 22304 Library of Congress Cataloging-in-Publication Data Bernstein, Lawrence R. Geology and mineralogy of the Apex Germanium Gallium mine, Washington County, Utah (U.S. Geological Survey Bulletin 1577) Bibliography: p. 9 Supt. of Docs. no.: I 19.3:1577 1. Mines and mineral resources-Utah-Washington County. 2. Mineralogy-Utah-Washington County. 3. Geology-Utah-Wasington County. I. Title. II. Series: United States. Geological Survey. Bulletin 1577. QE75.B9 no. 1577 557.3 s 85-600355 [TN24. U8] [553' .09792'48] CONTENTS Abstract 1 Introduction 1 Germanium and gallium 1 Apex Mine 1 Acknowledgments 3 Methods 3 Geologic setting 3 Regional geology 3 Local geology 3 Ore geology 4 Mineralogy 5 Primary ore 5 Supergene ore 5 Discussion and conclusions 7 Primary ore deposition 7. Supergene alteration 8 Implications 8 References 8 FIGURES 1. Map showing location of Apex Mine and generalized geology of surrounding region 2 2. Photograph showing main adit of Apex Mine and gently dipping beds of the Callville Limestone 3 3. Geologic map showing locations of Apex and Paymaster mines and Apex fault zone 4 4. Scanning electron photomicrograph showing plumbian jarosite crystals from the 1,601-m level, Apex Mine 6 TABLES 1. -

Duftite Pbcu(Aso4)(OH) C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic
Duftite PbCu(AsO4)(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 222. Crystals may be pseudo-octahedral, or elongated along [001], commonly curved and rough, to 5 mm; usually in crusts and aggregates. Physical Properties: Fracture: Conchoidal. Hardness = 4.5 D(meas.) = 6.40 D(calc.) = 6.602 Optical Properties: Subtranslucent. Color: Bright olive-green to gray-green; pale apple-green in transmitted light, generally zoned due to compositional variations. Streak: Pale green to white. Luster: Dull to vitreous on fractures. Optical Class: Biaxial (–). Dispersion: r> v,perceptible. α = 2.03–2.04 β = 2.06–2.08 γ = 2.08–2.10 2V(meas.) = Large. Cell Data: Space Group: P 212121 (synthetic). a = 7.768(1) b = 9.211(1) c = 5.999(1) Z=4 X-ray Powder Pattern: Tsumeb, Namibia. 3.26 (10), 2.85 (8), 2.65 (8), 2.57 (6), 4.21 (5), 2.28 (5), 1.87 (5) Chemistry: (1) (2) (3) (1) (2) (3) SiO2 0.44 CaO 0.75 0.9 + As2O5 26.01 26.1 26.93 H2O 2.65 − (Fe, Al)2O3 0.6 H2O 0.08 CuO 19.32 18.6 18.65 H2O 2.3 2.11 ZnO 0.46 0.7 Total 99.81 99.8 100.00 PbO 50.10 50.6 52.31 (1–2) Tsumeb, Namibia. (3) PbCu(AsO4)(OH). Mineral Group: Adelite group. Occurrence: An uncommon mineral in the oxidized zone of some hydrothermal base-metal deposits. Association: Olivenite, mottramite, azurite, malachite, wulfenite, calcite (Tsumeb, Namibia); bayldonite, beudantite, mimetite, cerussite (Cap Garonne mine, France). -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

The Crystal Chemistry of Duftite, Pbcuaso4(OH)
Mineralogical Magazine, February 1998, Vol. 62(1), pp. 121–130 The crystalchemistry of duftite, PbCuAsO4(OH) and the b-duftiteproblem KHARISUN*, MAX R. TAYLOR, D. J. M BEVAN Department of Chemistry, The Flinders University of South Australia, GPOBox 2100 Adelaide, S.A.5001, Australia AND ALLAN PRING Department of Mineralogy, South Australian Museum, North Terrace, S.A.5000, Australia ABSTRACT Duftite,PbCu(AsO 4)(OH)is orthorhombic, space group P212121 with a = 7.768(1), b = 9. 211(1), c = 5.999(1) AÊ ,Z=4;the structure has been refined to R =4.6 % and Rw = 6.5% using640 observed reflections[F> 2 s(F)].The structure consists of chainsof edge-sharingCuO 6 ‘octahedra’,parallelto c; whichare linked via AsO 4 tetrahedraand Pb atoms in distorted square antiprismatic co-ordination to forma threedimensional network. The CuO 6 ‘octahedra’ showJahn-Teller distortion with the elongationrunning approximately along <627>. The hydrogen bonding network in the structure was characterizedusing bond valence calculations. ‘b-duftite’ isan intermediate in the duftite– conichalcite series,which has a modulatedstructure based on the intergrowth of the two structures in domains of approximately50 A Ê .Theorigin of the modulation is thought to beassociatedwith displacements in the oxygenlattice and is related to the orientation of the Jahn-Teller distortion of CuO 6 ‘octahedra’. Approximatelyhalf of the strips show an elongation parallel to <627> while the other strips are elongatedparallel to [010 ].This ordering results in an increase in the b cellrepeat compared to duftite andconichalcite. KEY WORDS: duftite,conichalcite, crystal structure, crystal chemistry, modulated structure. hadearlier tentatively proposed the point group Introduction 222,on the basis of morphological observations. -

Joëlbruggerite, Pb3zn3(Sb5+,Te6+)
American Mineralogist, Volume 94, pages 1012–1017, 2009 5+ 6+ 5+ Joëlbruggerite, Pb3Zn3(Sb ,Te )As2O13(OH,O), the Sb analog of dugganite, from the Black Pine mine, Montana STUART J. MILLS ,1,* UWE KOLITSCH ,2 RITSURO MIYAWAKI ,3 LEE A. GROAT ,1 AND GLENN POIRIER 4 1Department of Earth and Ocean Sciences, University of British Columbia, 6339 Stores Road, Vancouver, British Columbia V6T 1Z4, Canada 2Mineralogisch-Petrographische Abteilung, Naturhistorisches Museum Wien, Burgring 7, A-1010 Wien, Austria 3Department of Geology, National Museum of Nature and Science, 3-23-1, Hyakunin-cho, Shinjuku, Tokyo 169-0073, Japan 4Mineral Sciences Division, Canadian Museum of Nature, P.O. Box 3443, Station D, Ottawa, Ontario K1P 6P4, Canada ABSTRACT 5+ 6+ Joëlbruggerite, ideally Pb3Zn3(Sb ,Te )As2O13(OH,O), is a new arsenate mineral (IMA 2008-034) and the Sb5+ analog of dugganite, from the Black Pine mine, 14.5 km northwest of Philipsburg, Granite County, Montana. It is usually found perched on mimetite; other species that may be present include malachite, azurite, pseudomalachite, chalcocite, beudantite-corkite, duftite, dugganite, and kuksite, in milky quartz veins. Joëlbruggerite occurs as barrel-shaped or prismatic crystals up to about 50 µm across in various shades of purple. The crystals have an adamantine luster and a white streak. Mohs hardness is about 3. The fracture is irregular, and the tenacity is brittle. Joëlbruggerite crystals are uniaxial (–), with a calculated refractive index of n = 1.993, and weakly pleochroic: X = Y = gray, Z = purple; absorption: Z > X = Y. Crystals show straight extinction and are length-fast. The empirical chemical formula (mean of 5 electron microprobe analyses) calculated on the basis of 14 [O + OH] 2+ 5+ 6+ anions is Pb3.112(Zn2.689Fe0.185)Σ2.874(Sb0.650Te 0.451)Σ1.101(As1.551P0.203Si0.160)Σ1.914O13.335(OH)0.665. -

Download PDF About Minerals Sorted by Mineral Group
MINERALS SORTED BY MINERAL GROUP Most minerals are chemically classified as native elements, sulfides, sulfates, oxides, silicates, carbonates, phosphates, halides, nitrates, tungstates, molybdates, arsenates, or vanadates. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). NATIVE ELEMENTS (DIAMOND, SULFUR, GOLD) Native elements are minerals composed of only one element, such as copper, sulfur, gold, silver, and diamond. They are not common in Kentucky, but are mentioned because of their appeal to collectors. DIAMOND Crystal system: isometric. Cleavage: perfect octahedral. Color: colorless, pale shades of yellow, orange, or blue. Hardness: 10. Specific gravity: 3.5. Uses: jewelry, saws, polishing equipment. Diamond, the hardest of any naturally formed mineral, is also highly refractive, causing light to be split into a spectrum of colors commonly called play of colors. Because of its high specific gravity, it is easily concentrated in alluvial gravels, where it can be mined. This is one of the main mining methods used in South Africa, where most of the world's diamonds originate. The source rock of diamonds is the igneous rock kimberlite, also referred to as diamond pipe. A nongem variety of diamond is called bort. Kentucky has kimberlites in Elliott County in eastern Kentucky and Crittenden and Livingston Counties in western Kentucky, but no diamonds have ever been discovered in or authenticated from these rocks. A diamond was found in Adair County, but it was determined to have been brought in from somewhere else. SULFUR Crystal system: orthorhombic. Fracture: uneven. Color: yellow. Hardness 1 to 2. -
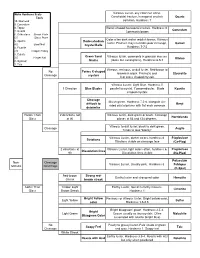
Mineral Flow Chart
Mohs Hardness Scale Vitreous Luster, any color can occur. Tools Conchoidal fracture, hexagonal crystals Quartz 10. Diamond common, Hardness 7 9. Corundum 8. Topaz Barrel shaped hexagonal crystals, Hardness 9, Corundum 7. Quartz Commonly brown 6. Orthoclase Streak Plate 5.5 Glass Plate Color often dark red or reddish brown. Vitreous 5. Apatite Dodecahedron luster. Fracture may resemble poor cleavage, Garnet 4.5 Steel Nail Crystal Balls 4. Fluorite Hardness 7-7.5 3.5 Copper Penny 3. Calcite Green Sand Vitreous luster, commonly in granular masses 2.5 Finger Nail Olivine 2. Gypsum Grains (looks like sand grains), Hardness 6.5-7 1. Talc Vitreous, resinous, or dull luster. Red-brown to No Forms X-shaped brownish-black. Prismatic and Staurolite Cleavage crystals X or cross shaped crystals Vitreous Luster, Light Blue. Hardness 5 1 Direction Blue Blades parallel to crystal, 7 perpendicular. Blade Kyanite shaped crystals Cleavage Bluish green. Hardness 7.5-8. Elongate six- difficult to Beryl sided crystal prisms with flat ends common determine Harder Than 2 directions not Vitreous luster, dark green or black. Cleavage Hornblende Glass at 90 planes at 56 and 124 degrees. Vitreous to dull luster, black to dark green. Cleavage Augite Tends to look "blocky" Vitreous Luster, darker colors, hardness 6. Plagioclase Striations Striations visible on cleavage face (Ca-Plag) 2 directions at Vitreous Luster, light colors often, hardness 6. Plagioclase Dissolution lines 90 Dissolution lines visible (Na-Plag) Potassium Non- Cleavage Vitreous Luster, Usually pink. Hardness 6. Feldspar Metallic Next Page (K-Spar) Red-brown Strong red- Earthy luster and strong red color Hematite Streak brown streak Softer Than Yellow/ Light Earthy Luster, found in earthy masses, Limonite Glass Brown Streak Hardness 1 Bright Yellow Resinous or vitreous luster.