Vol. 84 Thursday, No. 201 October 17, 2019 Pages 55489–55858
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sunday, October 4 Saturday, October 17
DUE TO COVID-19, ALL RESIDENTS MUST REMAIN IN THEIR VEHICLE AND PLACE THEIR MATERIALS IN THEIR TRUNK. Household Hazardous Waste, E-Waste & Tire Collection Days FREE! Sunday, October 4 Saturday, October 17 9AM – 1PM (RAIN OR SHINE) 9AM – 1PM (RAIN OR SHINE) Gorman Field in Bayonne Jersey City Municipal Service Complex Parking Lot – West 1st Street near Humphry Ave 13-15 Linden Ave Hoboken DPW Kearny DPW Willow Ave & Observer Highway 357 Bergen Ave WHAT TO BRING: WHAT NOT TO BRING: • Thermostats • Solvents & Thinners • Consumer Electronics • Infectious Waste • Tires • Pesticides • Alkaline Batteries • Silvex 2,4,5-t • Cleaners & Corrosives & Herbicides • Latex Paint • Radioactive Material • Pool & Photographic • Formaldehyde • Explosives • Unknown or Chemicals • Compact • Compressed Gas Unidentified Material • Oil Based Paints Fluorescent Bulbs Cylinders & Tanks • Ballasts & Varnishes • Used Motor Oil • TCBs, TCCD • Washers, Dryers, • Rechargeable • Old Gasoline (such as Freon & Helium) Refrigerators, & Car Batteries • Fire Extinguishers • Woodlife Air Conditioners • Propane Tanks • Antifreeze • Asbestos • Any OTC or (from BBQ grills only) • Smoke Detectors • Kepone Prescription Drugs TIRE AMNESTY COLLECTION COMPUTER & Hudson County residents are asked to bring no more than 4 tires for TECH RECYCLING proper disposal. If you cannot participate in this year’s Tire Amnesty Event, eitherstore tires indoors (in a garage or shed) or cover them until Computers, monitors, computer mice, keyboards, they can bedisposed of properly.* *CONTACT YOUR MUNICIPAL DPW FOR DISPOSAL LOCATIONS. tablets and cellphones can also be recycled. This program is sponsored, in part, by a grant from the NJDEP-Division of Solid and Hazardous Waste. Residents can drop off materials at any site. Proof of residency may be required. -

Early Dance Division Calendar 17-18
Early Dance Division 2017-2018 Session 1 September 9 – November 3 Monday Classes Tuesday Classes September 11 Class September 12 Class September 18 Class September 19 Class September 25 Class September 26 Class October 2 Class October 3 Class October 9 Class October 10 Class October 16 Class October 17 Class October 23 Class October 24 Class October 30 Last Class October 31 Last Class Wednesday Classes Thursday Classes September 13 Class September 14 Class September 20 Class September 21* Class September 27 Class September 28 Class October 4 Class October 5 Class October 11 Class October 12 Class October 18 Class October 19 Class October 25 Class October 26 Class November 1 Last Class November 2 Last Class Saturday Classes Sunday Classes September 9 Class September 10 Class September 16 Class September 17 Class September 23 Class September 24 Class September 30* Class October 1 Class October 7 Class October 8 Class October 14 Class October 15 Class October 21 Class October 22 Class October 28 Last Class October 29 Last Class *Absences due to the holiday will be granted an additional make-up class. Early Dance Division 2017-2018 Session 2 November 4 – January 22 Monday Classes Tuesday Classes November 6 Class November 7 Class November 13 Class November 14 Class November 20 No Class November 21 No Class November 27 Class November 28 Class December 4 Class December 5 Class December 11 Class December 12 Class December 18 Class December 19 Class December 25 No Class December 26 No Class January 1 No Class January 2 No Class January 8 Class -

Federal Register/Vol. 85, No. 202/Monday, October 19, 2020/Rules and Regulations
Federal Register / Vol. 85, No. 202 / Monday, October 19, 2020 / Rules and Regulations 66219 even if it is excluded from certain label SUMMARY: This document contains final hearing in response to that notice. On declarations. Finally, we reorganized regulations clarifying that the following August 5, 2020, the Treasury the section detailing our consideration deductions allowed to an estate or non- Department and the IRS published in of allulose as a sugar. grantor trust are not miscellaneous the Federal Register (85 FR 47323) a The guidance announced in this itemized deductions: Costs paid or cancellation of the notice of public notice finalizes the draft guidance with incurred in connection with the hearing. respect to: (1) Our views on the administration of an estate or non- The Treasury Department and the IRS declaration of allulose on Nutrition grantor trust that would not have been received written and electronic Facts and Supplement Facts labels and incurred if the property were not held comments in response to the proposed on the caloric content of allulose; and in the estate or trust, the personal regulations. All comments were (2) our intent to exercise enforcement exemption of an estate or non-grantor considered and are available at discretion for the exclusion of allulose trust, the distribution deduction for www.regulations.gov or upon request. from the amount of Total Sugars and trusts distributing current income, and After full consideration of the comments Added Sugars declared on the label and the distribution deduction for estates received, this Treasury decision adopts use of a general factor of 0.4 kcal/g for and trusts accumulating income. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
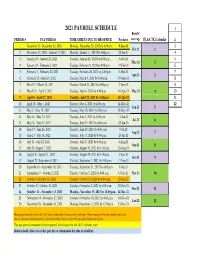
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -
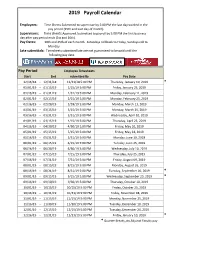
2019 Payroll Calendar
2019 Payroll Calendar Employees: Time Sheets Submitted to supervisor by 5:00 PM the last day worked in the pay period (15th and Last day of month). Supervisors: Time sheets Approved /submitted to payroll by 5:00 PM the first business day after pay period ends (1st and 16th). Pay Dates: 10th and 25th of each month. Saturdays roll back to Friday, Sundays roll to Monday. Late submittals: Timesheets submitted late are not guaranteed to be paid until the following pay date. Pay Period Employee Timesheets Start End submitted By Pay Date 12/16/18 - 12/31/18 12/31/18 5:00 PM Thursday, January 10, 2019 * 01/01/19 - 01/15/19 1/15/19 5:00 PM Friday, January 25, 2019 01/16/19 - 01/31/19 1/31/19 5:00 PM Monday, February 11, 2019 02/01/19 - 02/15/19 2/15/19 5:00 PM Monday, February 25, 2019 02/16/19 - 02/28/19 2/28/19 5:00 PM Monday, March 11, 2019 03/01/19 - 03/15/19 3/15/19 5:00 PM Monday, March 25, 2019 03/16/19 - 03/31/19 3/31/19 5:00 PM Wednesday, April 10, 2019 04/01/19 - 04/15/19 4/15/19 5:00 PM Thursday, April 25, 2019 04/16/19 - 04/30/19 4/30/19 5:00 PM Friday, May 10, 2019 05/01/19 - 05/15/19 5/15/19 5:00 PM Friday, May 24, 2019 05/16/19 - 05/31/19 5/31/19 5:00 PM Monday, June 10, 2019 06/01/19 - 06/15/19 6/15/19 5:00 PM Tuesday, June 25, 2019 06/16/19 - 06/30/19 6/30/19 5:00 PM Wednesday, July 10, 2019 07/01/19 - 07/15/19 7/15/19 5:00 PM Thursday, July 25, 2019 07/16/19 - 07/31/19 7/31/19 5:00 PM Friday, August 09, 2019 08/01/19 - 08/15/19 8/15/19 5:00 PM Monday, August 26, 2019 08/16/19 - 08/31/19 8/31/19 5:00 PM Tuesday, September 10, -

FOR RELEASE October 17, 2019 for MEDIA OR
FOR RELEASE October 17, 2019 FOR MEDIA OR OTHER INQUIRIES: Carroll Doherty, Director of Political Research Jocelyn Kiley, Associate Director, Research Nida Asheer, Communications Associate 202.419.4372 www.pewresearch.org RECOMMENDED CITATION Pew Research Center, October 2019, “Modest Changes in Views of Impeachment Proceedings Since Early September” 1 PEW RESEARCH CENTER About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It does not take policy positions. The Center conducts public opinion polling, demographic research, content analysis and other data-driven social science research. It studies U.S. politics and policy; journalism and media; internet, science and technology; religion and public life; Hispanic trends; global attitudes and trends; and U.S. social and demographic trends. All of the Center’s reports are available at www.pewresearch.org. Pew Research Center is a subsidiary of The Pew Charitable Trusts, its primary funder. © Pew Research Center 2019 www.pewresearch.org 2 PEW RESEARCH CENTER Most Americans have not changed their views on whether the House should conduct impeachment proceedings against President Donald Trump since early September, before House Speaker Nancy Pelosi announced that the House would conduct an impeachment inquiry of the president. Majority of public approves of House But about one-in-ten adults (9%) who had decision to begin impeachment inquiry opposed the House opening impeachment % who ___ of the House of Representatives’ decision to proceedings last month now approve of the begin an impeachment inquiry decision to conduct an impeachment inquiry, based on an analysis that tracks change in opinion among the same survey respondents How confident are you that ____ in Congress will be over time. -

COVID-19 Dashboard- Monday, October 19, 2020 Dashboard of Public Health Indicators
10/19/2020 Public Health Indicators Massachusetts Department of Public Health COVID-19 Dashboard- Monday, October 19, 2020 Dashboard of Public Health Indicators Newly Reported Total Confirmed Newly Reported Total Deaths Confirmed Cases Cases Deaths among among Confirmed Today Confirmed Today Cases 827 141,474 15 9,532 New Individuals Total Individuals Below is the current status: Tested by Tested by Molecular Tests Molecular Tests Measure Status 17,654 2,532,287 COVID-19 positive test rate ⚫ Number of individuals who died from COVID-19 ⚫ Total Molecular Legend Number of patients with COVID-19 in hospitals ⚫ Tests Healthcare system readiness ⚫ Administered Testing capacity ⚫ 5,239,651 Contact tracing capabilities ⚫ The front page of the dashboard has been reformatted. Antibody tests (individual and total numbers) can be found on page 18. 1 1/1 10/19/2020 Public Health Indicators2 Massachusetts Department of Public Health COVID-19 Dashboard- Monday, October 19, 2020 Percent or Count of Change Since Dashboard of Public Health Indicators Lowest Observed Value (LOV) 1.4% 7 Day Weighted 1.2% 1.2% 1.2% 1.1% 1.1% 1.1% 1.1% 1.2% 1.1% 1.1% 1.1% Average of Positive 1.2% 1.1% 1.3% 1.3% 1.2% 0.9% 1.0% 1.2% 1.3% 1.3% 1.2% 1.0% 0.9% 0.9% 1.1% 59 % Molecular Test Rate* 0.8% 0.8% 0.8% 0.8% 1.1% 0.8% 0.6% 0.8% 19 20 21 22 23 24 25 26 27 28 29 30 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 September October LOV =0.8% 3 Day Average of 494 498 500 499 Number of COVID- 500 468 439 442 514 514 423 433 432 425 505 509 505 505 505 505 494 19 Patients in 393 -

BILLING CYCLE SCHEDULE Department of Procurement, Disbursements & Contract Services 1135 Old Main 600 Lincoln Avenue Charleston, IL 61920
Eastern Illinois University BILLING CYCLE SCHEDULE Department of Procurement, Disbursements & Contract Services 1135 Old Main 600 Lincoln Avenue Charleston, IL 61920 Billing Cycle Beginning Date Billing Cycle Ending Date June 26, 2018 July 25, 2018 Tuesday Wednesday July 26, 2018 August 24, 2018 Thursday Friday August 26, 2018 September 25, 2018 Sunday Tuesday September 26, 2018 October 25, 2018 Wednesday Thursday October 26, 2018 November 26, 2018 Friday Monday November 27, 2018 December 26, 2018 Tuesday Wednesday December 27, 2018 January 25, 2019 Thursday Friday January 26, 2019 February 25, 2019 Saturday Monday February 26, 2019 March 25, 2019 Tuesday Monday March 26, 2019 April 25, 2019 Tuesday Thursday April 26, 2019 May 24, 2019 Friday Friday May 26, 2019 June 25, 2019 Sunday Tuesday June 26, 2019 July 25, 2019 Wednesday Thursday Revised 2/2/18 1 Transactions with a Post Date of: Must be Reviewed Upload to Banner & Approved by: July 1, 2018 – July 6, 2018 July 12, 2018 July 13, 2018 Thursday Friday July 7, 2018 – July 13, 2018 July 19, 2018 July 20, 2018 Thursday Friday July 14, 2018 – July 20, 2018 July 26, 2018 July 27, 2018 Thursday Friday July 21, 2018 – July 27, 2018 August 2, 2018 August 3, 2018 Thursday Friday July 28, 2018 – August 3, 2018 August 9, 2018 August 10, 2018 Thursday Friday August 4, 2018 – August 10, 2018 August 16, 2018 August 17, 2018 Thursday Friday August 11, 2018 – August 17, 2018 August 23, 2018 August 24, 2018 Thursday Friday August 18, 2018 – August 24, 2018 August 30, 2018 August 31, 2018 Thursday -

Salary Payroll Schedule - 2021 Fiscal Pay Salary Overtime &Retro Leave Semester Pay Period HR Transaction Deadline Payday Days Year Number Entry (PHAHOUR) Periods
Salary Payroll Schedule - 2021 Fiscal Pay Salary Overtime &Retro Leave Semester Pay Period HR Transaction deadline Payday Days Year Number Entry (PHAHOUR) Periods December 25 – January 9 1 Monday, January 4, 2021 Wednesday, January 6, 2021 Friday, January 15, 2021 11 12 January 10 – January 24 2 Tuesday, January 19, 2021 Thursday, January 21, 2021 Monday, February 1, 2021 10 1 January 25 – February 9 3 Tuesday, February 2, 2021 Thursday, February 4, 2021 Tuesday, February 16, 2021 12 February 10 – February 24 4 Tuesday, February 16, 2021 Thursday, February 18, 2021 Monday, March 1, 2021 11 Spring Semester 2 Classes Begin February 25 – March 9 5 Wednesday, March 3, 2021 Friday, March 5, 2021 Tuesday, March 16, 2021 9 1/19/21 Exams End 5/13/21 21 March 10 – March 24 6 Thursday, March 18, 2021 Monday, March 22, 2021 Wednesday, March 31, 2021 11 3 March 25 – April 9 7 Monday, April 5, 2021 Wednesday, April 7, 2021 Friday, April 16, 2021 12 April 10 – April 24 8 Monday, April 19, 2021 Wednesday, April 21, 2021 Friday, April 30, 2021 10 4 April 25 – May 9 9 Monday, May 3, 2021 Wednesday, May 5, 2021 Friday, May 14, 2021 10 May 10 – May 24 10 Tuesday, May 18, 2021 Thursday, May 20, 2021 Tuesday, June 1, 2021 11 Summer I 5 Classes Begin May 25 – June 9 11 Thursday, June 3, 2021 Monday, June 7, 2021 Wednesday, June 16, 2021 12 5/24/21 (Paid 7/1/21) June 10 – June 24 12 Friday, June 18, 2021 Tuesday, June 22, 2021 Thursday, July 1, 2021 11 6 June 25 – July 9 13 Friday, July 2, 2021 Wednesday, July 7, 2021 Friday, July 16, 2021 11 Summer II Classes -
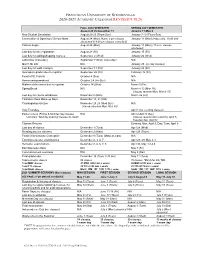
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -

2021 Daily 10 October
October 2021 Daily Lectionary These Scripture readings are from the two-year daily lectionary of the Presbyterian Book of Common Worship (Westminster John Knox Press, 2018). The readings from the three-year Revised Common Lectionary for Sundays and Festivals are not included in this document. Friday, October 1 Thursday, October 7 Wednesday, October 13 Morning: Pss. 84; 148 Morning: Pss. 97; 147:12–20 Morning: Pss. 15; 147:1–11 Evening: Pss. 25; 40 Evening: Pss. 16; 62 Evening: Pss. 48; 4 2 Kings 19:1–20 2 Kings 23:4–25 Jer. 37:3–21 1 Cor. 9:16–27 1 Cor. 12:1–11 1 Cor. 14:13–25 Matt. 8:1–17 Matt. 9:18–26 Matt. 10:24–33 Saturday, October 2 Friday, October 8 Thursday, October 14 Morning: Pss. 63; 149 Morning: Pss. 51; 148 Morning: Pss. 36; 147:12–20 Evening: Pss. 125; 90 Evening: Pss. 142; 65 Evening: Pss. 80; 27 2 Kings 19:21–36 2 Kings 23:36–24:17 Jer. 38:1–13 1 Cor. 10:1–13 1 Cor. 12:12–26 1 Cor. 14:26–33a Matt. 8:18–27 Matt. 9:27–34 (33b–36) 37–40 Matt. 10:34–42 Sunday, October 3 Saturday, October 9 Morning: Pss. 103; 150 Morning: Pss. 104; 149 Friday, October 15 Evening: Pss. 117; 139 Evening: Pss. 138; 98 Morning: Pss. 130; 148 2 Kings 20:1–21 Jer. 35:1–19 Evening: Pss. 32; 139 Acts 12:1–17 1 Cor. 12:27–13:3 Jer. 38:14–28 Luke 7:11–17 Matt.