Department of Consumer Affairs California State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U.S. House of Representatives Committee on Energy and Commerce
U.S. HOUSE OF REPRESENTATIVES COMMITTEE ON ENERGY AND COMMERCE December 8, 2016 TO: Members, Subcommittee on Commerce, Manufacturing, and Trade FROM: Committee Majority Staff RE: Hearing entitled “Mixed Martial Arts: Issues and Perspectives.” I. INTRODUCTION On December 8, 2016, at 10:00 a.m. in 2322 Rayburn House Office Building, the Subcommittee on Commerce, Manufacturing, and Trade will hold a hearing entitled “Mixed Martial Arts: Issues and Perspectives.” II. WITNESSES The Subcommittee will hear from the following witnesses: Randy Couture, President, Xtreme Couture; Lydia Robertson, Treasurer, Association of Boxing Commissions and Combative Sports; Jeff Novitzky, Vice President, Athlete Health and Performance, Ultimate Fighting Championship; and Dr. Ann McKee, Professor of Neurology & Pathology, Neuropathology Core, Alzheimer’s Disease Center, Boston University III. BACKGROUND A. Introduction Modern mixed martial arts (MMA) can be traced back to Greek fighting events known as pankration (meaning “all powers”), first introduced as part of the Olympic Games in the Seventh Century, B.C.1 However, pankration usually involved few rules, while modern MMA is generally governed by significant rules and regulations.2 As its name denotes, MMA owes its 1 JOSH GROSS, ALI VS.INOKI: THE FORGOTTEN FIGHT THAT INSPIRED MIXED MARTIAL ARTS AND LAUNCHED SPORTS ENTERTAINMENT 18-19 (2016). 2 Jad Semaan, Ancient Greek Pankration: The Origins of MMA, Part One, BLEACHERREPORT (Jun. 9, 2009), available at http://bleacherreport.com/articles/28473-ancient-greek-pankration-the-origins-of-mma-part-one. -
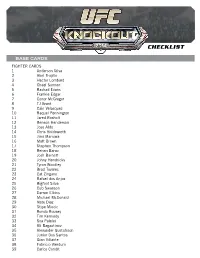
2014 Topps UFC Knockout Checklist
CHECKLIST BASE CARDS FIGHTER CARDS 1 Anderson Silva 2 Abel Trujillo 3 Hector Lombard 4 Chael Sonnen 5 Rashad Evans 6 Frankie Edgar 7 Conor McGregor 8 TJ Grant 9 Cain Velasquez 10 Raquel Pennington 11 Jared Rosholt 12 Benson Henderson 13 Jose Aldo 14 Chris Holdsworth 15 Jimi Manuwa 16 Matt Brown 17 Stephen Thompson 18 Renan Barao 19 Josh Barnett 20 Johny Hendricks 21 Tyron Woodley 22 Brad Tavares 23 Cat Zingano 24 Rafael dos Anjos 25 Bigfoot Silva 26 Cub Swanson 27 Darren Elkins 28 Michael McDonald 29 Nate Diaz 30 Stipe Miocic 31 Ronda Rousey 32 Tim Kennedy 33 Soa Palelei 34 Ali Bagautinov 35 Alexander Gustafsson 36 Junior Dos Santos 37 Gian Villante 38 Fabricio Werdum 39 Carlos Condit CHECKLIST 40 Brandon Thatch 41 Eddie Wineland 42 Pat Healy 43 Roy Nelson 44 Myles Jury 45 Chad Mendes 46 Nik Lentz 47 Dustin Poirier 48 Travis Browne 49 Glover Teixeira 50 James Te Huna 51 Jon Jones 52 Scott Jorgensen 53 Santiago Ponzinibbio 54 Ian McCall 55 George Roop 56 Ricardo Lamas 57 Josh Thomson 58 Rory MacDonald 59 Edson Barboza 60 Matt Mitrione 61 Ronaldo Souza 62 Yoel Romero 63 Alexis Davis 64 Demetrious Johnson 65 Vitor Belfort 66 Liz Carmouche 67 Julianna Pena 68 Phil Davis 69 TJ Dillashaw 70 Sarah Kaufman 71 Mark Munoz 72 Miesha Tate 73 Jessica Eye 74 Steven Siler 75 Ovince Saint Preux 76 Jake Shields 77 Chris Weidman 78 Robbie Lawler 79 Khabib Nurmagomedov 80 Frank Mir 81 Jake Ellenberger CHECKLIST 82 Anthony Pettis 83 Erik Perez 84 Dan Henderson 85 Shogun Rua 86 John Makdessi 87 Sergio Pettis 88 Urijah Faber 89 Lyoto Machida 90 Demian Maia -

BAKER COUNTY BOARD of COUNTY COMMISSIONERS AGENDA AUGUST 4, 2020 MEETING CONNECTION INFORMATION: to Access the Meeting Please U
BAKER COUNTY BOARD OF COUNTY COMMISSIONERS AGENDA AUGUST 4, 2020 **This meeting will be conducted in a virtual environment due to COVID-19 and in accordance with State of Florida Executive Order 20-69. Please see the attached procedures for participating in this meeting. **All written comments submitted to the Commission through e-mail or the Zoom Chat function will be distributed to each Commissioner and also be included with the public record, but will not be read aloud during the meeting. Anyone wishing to speak will be given the opportunity during the PUBLIC COMMENT section of the agenda as part of the virtual meeting through Zoom. MEETING CONNECTION INFORMATION: To access the meeting please use the following: Web Access: https://us02web.zoom.us/j/84633159918 Telephone Access: 1-301-715-8592 Secondary Access Line: 1-312-626-6799 Meeting ID: 846 3315 9918 REGULAR SESSION 5:00 P.M. I. INVOCATION AND PLEDGE OF ALLEGIANCE II. APPROVAL OF AGENDA III. APPROVAL OF CONSENT AGENDA ITEMS 1. Minutes- July 21, 2020 – Regular Session 2. Minutes – July 21, 2020 – Public Hearing 3. Expense Report 4. Request to Advertise – Community Development Director 5. Acceptance of Property from LaBuena Farms for Midpoint Pkwy 6. Confirmation of CDBG-MIT Grant application 7. Approval of County Manager Contract IV. PUBLIC COMMENTS All public comments should be made at this time. Not as each item is discussed. V. CONSTITUTIONAL OFFICERS VI. NEW BUSINESS 1. Approval FDOT Supplemental Agreement for CR 23A; Robert Fletcher Action Item 2. Award BID 2020-06 for CR 23A; Robert Fletcher Action Item 3. -

Chael Sonnen Joins World Series of Fighting As Color Commentator for Live Nbcsn Telecasts
CHAEL SONNEN JOINS WORLD SERIES OF FIGHTING AS COLOR COMMENTATOR FOR LIVE NBCSN TELECASTS LAS VEGAS (September 1, 2015) –Mixed Martial Arts (MMA) superstar Chael Sonnen has signed on with World Series of Fighting (WSOF.com) as a color commentator for multiple events, and will call his first action with veteran play-by- play announcer Todd Harris, at the highly-anticipated “WSOF 23: Gaethje vs. Palomino II” world championship doubleheader mega-event, live on NBCSN from Phoenix, Ariz. on Friday, Sept. 18 at 10 p.m. ET. “I am stoked to join the powerhouse team at World Series of Fighting, and call their fights that feature some of the best mixed martial artists on the planet,” said Sonnen “World Series of Fighting has done an outstanding job of building their organization into a top-tier franchise in a very short span of time, and I am just proud to be a part of such a fast- growing company. “We are thrilled to have Chael Sonnen, one of the most colorful individuals in professional sports, join our production,” said World Series of Fighting President Ray Sefo. “We are confident Chael will enhance the fan viewing experience both for our growing national audience on NBCSN and across the globe by providing the insights he learned competing at the highest level of the sport.” The 38-year-old Sonnen is a former three-time UFC world championship challenger, NCAA Division I All-American wrestler and two-time PAC-10 runner-up for the University of Oregon and 2000 Greco-Roman World University championships silver medalist. -

Celebrating Cimarron a History in by Beverly Ponterio Staff Writer by Garett Franklyn Progress Staff Writer
I N BACKCOUNTRY CAMP FEATURES BACKCOUNTRY COOKING RECIPES STAFF HIGHLIGHT PEACHES S PAGES 7-9 PAGE 10 PAGE 23 I D E PhilmontScoutRanch.org June 29, 2012 Issue 4 PhilNewsCelebrating Cimarron A History in By Beverly Ponterio Staff Writer By Garett Franklyn Progress Staff Writer Visitors peruse the historic artifacts on the walls of the St. James Hotel on Saturday, June 23, 2012. ERIN NASH/PHILNEWS PHOTOGRAPHER A tarnished bronze register what it’s changed into.” sits at the front desk of the St. It’s a history that began in David VanDeValdy, a traditional woodsmith, handcrafts household items such as kitchen utensils and James Hotel, a $5.01 still tallied 1872 when the hotel was built by benches. He traveled from Texas to share his trade. LYNN DECAPO/PHILNEWS PHOTOGRAPHER on the display from the last Henri Lambert, who was once The sky was an incredible with bone handles. Everything hand using traditional old tools. fingers that punched them in. the personal chef to President blue as the sun beat down on the seemed homemade and some At his tent, VanDeValdy Nearby is the more modern and Lincoln. Since then, it’s been vendors’ tents Saturday morning were made right in front of you was set up and making cooking functioning one worked by the the host not only of guests, but at Cimarron Days. There was as you walked around. utensils, a labor of love for him. receptionist. gunfights, gambling and maybe music playing over loudspeakers One Texan vendor, David He said, while utensils are the The contrast between old even ghosts. -
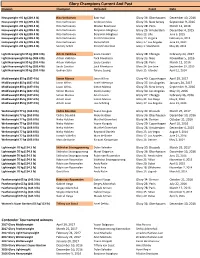
Glory Champions Current and Past Division Champion Defeated Event Date
Glory Champions Current And Past Division Champion Defeated Event Date Heavyweight +95 kg (209.4 lb) Rico Verhoeven Badr Hari Glory 36: Oberhausen December 10, 2016 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Anderson Silva Glory 33: New Jersey September 9, 2016 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Mladen Brestovac Glory 28: Paris March 12, 2016 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Benjamin Adegbuyi Glory 26: Amsterdam December 4, 2015 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Benjamin Adegbuyi Glory 22: Lille June 5, 2015 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Errol Zimmerman Glory 19: Virginia February 6, 2015 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Daniel Ghiță Glory 17: Los Angeles June 21, 2014 Heavyweight +95 kg (209.4 lb) Semmy Schilt Errol Zimmerman Glory 1: Stockholm May 26, 2012 Light Heavyweight 95 kg (209.4 lb) Artem Vakhitov Saulo Cavalari Glory 38: Chicago February 24, 2017 Light Heavyweight 95 kg (209.4 lb) Artem Vakhitov Zack Mwekassa Glory 35: Nice November 5, 2016 Light Heavyweight 95 kg (209.4 lb) Artem Vakhitov Saulo Cavalari Glory 28: Paris March 12, 2016 Light Heavyweight 95 kg (209.4 lb) Saulo Cavalari Zack Mwekassa Glory 24: San Jose September 19, 2015 Light Heavyweight 95 kg (209.4 lb) Gökhan Saki Tyrone Spong Glory 15: Istanbul April 12, 2014 Middleweight 85 kg (187.4 lb) Simon Marcus Jason Wilnis Glory 40: Copenhagen April 29, 2017 Middleweight 85 kg (187.4 lb) Jason Wilnis Israel Adesanya Glory 37: Los Angeles January 20, 2017 Middleweight 85 kg (187.4 lb) Jason Wilnis Simon Marcus -

Are Ufc Fighters Employees Or Independent Contractors?
Conklin Book Proof (Do Not Delete) 4/27/20 8:42 PM TWO CLASSIFICATIONS ENTER, ONE CLASSIFICATION LEAVES: ARE UFC FIGHTERS EMPLOYEES OR INDEPENDENT CONTRACTORS? MICHAEL CONKLIN* I. INTRODUCTION The fighters who compete in the Ultimate Fighting Championship (“UFC”) are currently classified as independent contractors. However, this classification appears to contradict the level of control that the UFC exerts over its fighters. This independent contractor classification severely limits the fighters’ benefits, workplace protections, and ability to unionize. Furthermore, the friendship between UFC’s brash president Dana White and President Donald Trump—who is responsible for making appointments to the National Labor Relations Board (“NLRB”)—has added a new twist to this issue.1 An attorney representing a former UFC fighter claimed this friendship resulted in a biased NLRB determination in their case.2 This article provides a detailed examination of the relationship between the UFC and its fighters, the relevance of worker classifications, and the case law involving workers in related fields. Finally, it performs an analysis of the proper classification of UFC fighters using the Internal Revenue Service (“IRS”) Twenty-Factor Test. II. UFC BACKGROUND The UFC is the world’s leading mixed martial arts (“MMA”) promotion. MMA is a one-on-one combat sport that combines elements of different martial arts such as boxing, judo, wrestling, jiu-jitsu, and karate. UFC bouts always take place in the trademarked Octagon, which is an eight-sided cage.3 The first UFC event was held in 1993 and had limited rules and limited fighter protections as compared to the modern-day events.4 UFC 15 was promoted as “deadly” and an event “where anything can happen and probably will.”6 The brutality of the early UFC events led to Senator John * Powell Endowed Professor of Business Law, Angelo State University. -

2015 Topps UFC Chronicles Checklist
BASE FIGHTER CARDS 1 Royce Gracie 2 Gracie vs Jimmerson 3 Dan Severn 4 Royce Gracie 5 Don Frye 6 Vitor Belfort 7 Dan Henderson 8 Matt Hughes 9 Andrei Arlovski 10 Jens Pulver 11 BJ Penn 12 Robbie Lawler 13 Rich Franklin 14 Nick Diaz 15 Georges St-Pierre 16 Patrick Côté 17 The Ultimate Fighter 1 18 Forrest Griffin 19 Forrest Griffin 20 Stephan Bonnar 21 Rich Franklin 22 Diego Sanchez 23 Hughes vs Trigg II 24 Nate Marquardt 25 Thiago Alves 26 Chael Sonnen 27 Keith Jardine 28 Rashad Evans 29 Rashad Evans 30 Joe Stevenson 31 Ludwig vs Goulet 32 Michael Bisping 33 Michael Bisping 34 Arianny Celeste 35 Anderson Silva 36 Martin Kampmann 37 Joe Lauzon 38 Clay Guida 39 Thales Leites 40 Mirko Cro Cop 41 Rampage Jackson 42 Frankie Edgar 43 Lyoto Machida 44 Roan Carneiro 45 St-Pierre vs Serra 46 Fabricio Werdum 47 Dennis Siver 48 Anthony Johnson 49 Cole Miller 50 Nate Diaz 51 Gray Maynard 52 Nate Diaz 53 Gray Maynard 54 Minotauro Nogueira 55 Rampage vs Henderson 56 Maurício Shogun Rua 57 Demian Maia 58 Bisping vs Evans 59 Ben Saunders 60 Soa Palelei 61 Tim Boetsch 62 Silva vs Henderson 63 Cain Velasquez 64 Shane Carwin 65 Matt Brown 66 CB Dollaway 67 Amir Sadollah 68 CB Dollaway 69 Dan Miller 70 Fitch vs Larson 71 Jim Miller 72 Baron vs Miller 73 Junior Dos Santos 74 Rafael dos Anjos 75 Ryan Bader 76 Tom Lawlor 77 Efrain Escudero 78 Ryan Bader 79 Mark Muñoz 80 Carlos Condit 81 Brian Stann 82 TJ Grant 83 Ross Pearson 84 Ross Pearson 85 Johny Hendricks 86 Todd Duffee 87 Jake Ellenberger 88 John Howard 89 Nik Lentz 90 Ben Rothwell 91 Alexander Gustafsson -

(+232 Lbs ) KINGS of the RING WORLD SERIES
OPEN SUPER HEAVYWEIGHT +105 kg (+232 lbs ) Japanese boxing - Shootboxing rules Super world champion Date, Place VACANT 14 World champion Date, Place VACANT Thai boxing - Full muaythai rules Thai boxing - International muaythai rules SUPER WORLD CHAMPION Date, Place SUPER WORLD CHAMPION Date, place VACANT VACANT WORLD CHAMPION Date, Place WORLD CHAMPION Date, Place 09.02.2008 LLOYD VAN DAMS ( NL ) 29.05.2004 TONY GREGORY ( FRA ) Auckland- = 289 pts Venice-ITA = 413 pts NZ OPBU EURO-AFRICAN CHAMPION Date, Place OPBU EURO-AFRICAN CHAMPION Date, Place VACANT VACANT Japanese boxing - K -1 rules Japanese boxing - Oriental Kick rules SUPER WORLD CHAMPION Date, Place SUPER WORLD CHAMPION Date, Place VACANT VACANT WORLD CHAMPION Date, Place WORLD CHAMPION Date, Place VACANT VACANT OPBU EURO-AFRICAN CHAMPION Date, Place OPBU EURO-AFRICAN CHAMPION Date, Place VACANT VACANT KINGS OF THE RING WORLD SERIES WIPU ORIENTAL PRO BOXING RULES SUPER WORLD CHAMPION Date, place VACANT e-mail : [email protected] mobile phone : +385 98 421 300 www.wipu-kings.com OPEN SUPER HEAVYWEIGHT +105 kg (+232 lbs ) 1. Semmy Schilt ( NL ) = 964 pts 2. Peter Aerts ( NL ) = 645 pts 3. Jerome Lebanner ( FRA ) = 571 pts 4. Alistar Overeem ( NL ) = 524 pts 5. Alexei Ignashov ( BLR ) = 432 pts 6. Daniel Ghita ( ROM ) = 362 pts 7. '' MIGHTY MO '' Siligia ( USA ) = 362 pts 8. Anderson '' Bradock '' Silva ( BRA ) = 344 pts 9. Mladen Brestovac ( CRO ) = 311 pts 10. Ismael Londt ( NL ) = 290 pts 11. Rico Verhoeven ( NL ) = 275 pts 12. Ben Edwards ( AUS ) = 258 pts 13. Peter Graham ( AUS ) = 243 pts 14. Alexandre Pitchkounov ( RUS ) = 236 pts 15. -

Business Process Management & Enterprise Architecture Track
BIO - Bioinformatics Track Track Co-Chairs: Juan Manuel Corchado, University of Salamanca, Spain Paola Lecca, University of Trento, Italy Dan Tulpan, University of Guelph, Canada An Insight into Biological Data Mining based on Rarity and Correlation as Constraints .............................1 Souad Bouasker, University of Tunis ElManar, Tunisia Drug Target Discovery Using Knowledge Graph Embeddings .........................................................................9 Sameh K. Mohamed, Insight Centre for Data Analytics, Ireland Aayah Nounu, University of Bristol, UK Vit Novacek, INSIGHT @ NUI Galway, Ireland Ensemble Feature Selectin for Biomarker Discovery in Mass Spectrometry-based Metabolomics ............17 Aliasghar Shahrjooihaghighi, University of Louisville, USA Hichem Frigui, University of Louisville, USA Xiang Zhang, University of Louisville, USA Xiaoli Wei, University of Louisville, USA Biyun Shi, University of Louisville, USA Craig J. McClain, University of Louisville, USA Molecule Specific Normalization for Protein and Metabolite Biomarker Discovery ....................................23 Ameni Trabelsi, University of Louisville, USA Biyun Shi, University of Louisville, USA Xiaoli Wei, University of Louisville, USA HICHEM FRIGUI, University of Louisville, USA Xiang Zhang, University of Louisville, USA Aliasghar Shahrajooihaghighi, University of Louisville, USA Craig McClain, University of Louisville, USA BPMEA - Business Process Management & Enterprise Architecture Track Track Co-Chairs: Marco Brambilla, Politecnico di -

Help for Homeless Peace Events Change Hearts
Lampanelli GTAV review ‘Quean of Mean’ Hottest new game smack talks Orlando sets new records page 14 page 15 Volume 19, Issue 3 www.ValenciaVoice.com Sept. 25, 2013 Peace events change hearts Valencia East Campus gives students a chance to spread love By Christopher Stanley [email protected] EAST CAMPUS — Carl Wilkens -- author of “I’m Not Leaving,” an ac- count of his time in Rwanda during the Azabache Films. genocide in 1994 -- finished Peace Day last Thursday with his presentation, “Help Build a Peaceful World by Seeing the ‘Other’ as Yourself.” Help for homeless “I don’t think horror is a lasting mo- tivator,” Wilkens said to a packed room. Film looks at problem in heart of Orlando “It’s love, plain and simple.” Peace Day was part of Peace Week By Stephanie Cardenas on the streets. Instead it isn’t unusual to 2013, sponsored by the Peace and Jus- [email protected] pass by a homeless person and ignore tice Initiative (PJI). Several tables were them as if they are invisible. An invis- set up at Valencia East Campus’ Mall WEST CAMPUS — What is the first ible homeless is overlooked by the pub- area with pamphlets advertising the thing that pops into your mind when lic and often not regarded as a priority of student group and opportunities for you see a homeless person on the streets? any means. These are the people whose passing students to write a letter to Syr- Drug users, alcoholics, physiological name is not a name, homeless. ian refugees, write what the word peace problems; these are the common miscon- Valencia College West Campus had means to them or gather information on ception most people believe to be true. -

25 Pro Fighters, Managers, and Coaches Reveal Their Best Tips to Land a Sponsorship by Solmadrid Vazquez Follow Me on Twitter Here
25 Pro Fighters, Managers, and Coaches Reveal Their Best Tips to Land a Sponsorship by Solmadrid Vazquez Follow me on Twitter here. Sponsorships can make or break you. The problem is, the process of landing a sponsorship is counter-intuitive. Being a great fighter is NOT enough. I’m sure you’ve seen fighters who land sponsors left and right. What’s their secret? How come they can get 27 sponsors in one day and you can’t even get one freakin’ rep to look at you? What THE hell is going on?! To get to this bottom of this conundrum, I contacted some of the best fighters, managers, and trainers in the game and asked them a simple question: “What is your #1 tip to land a sponsorship?” Each tip has a custom tweet link after it so feel free to share your favorite tips with your followers. Let’s Get Ready To Ruuuummmmbllllllleee!!! Frank Shamrock Frank Shamrock is a retired MMA Fighter. He was the first UFC Middleweight Champion and retired as the four-time defending undefeated champion. He was also the first WEC Light Heavyweight Champion, and the first Strikeforce Middleweight Champion. He was a brand spokesman for Strikeforce and is a Sports Commentator for Showtime. Frank can be found at his site, on Facebook, and on Twitter. My number one tip to landing a sponsorship is presenting yourself properly. Present a long-term consistent growth plan that somebody, or a sponsor, could attach themselves to, so you can show how you will grow together. “Present a long-term consistent growth plan.” Tweet this.