Large-Scale Brain Networks in Cognition: Emerging Methods and Principles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring the Sensorimotor Network Using Functional Connectivity and Graph Theory
EXPLORING THE SENSORIMOTOR NETWORK USING FUNCTIONAL CONNECTIVITY AND GRAPH THEORY by Ronald Bishop Submitted in partial fulfillment of the requirements for the degree of Master of Science at Dalhousie University Halifax, Nova Scotia August 2014 © Copyright by Ronald Bishop, 2014 TABLE OF CONTENTS LIST OF TABLES ......................................................................................................................................... v LIST OF FIGURES ...................................................................................................................................... vi ABSTRACT ................................................................................................................................................. vii LIST OF ABBREVIATIONS USED ......................................................................................................... viii ACKNOWLEDGEMENTS ......................................................................................................................... xi CHAPTER 1: INTRODUCTION ................................................................................................................. 1 CHAPTER 2: LITERATURE REVIEW ..................................................................................................... 4 2.1 AN OVERVIEW OF NEURONAL COMMUNICATION ..................................................................................... 4 2.2 SYNCHRONOUS NEURAL FIRING .............................................................................................................. -
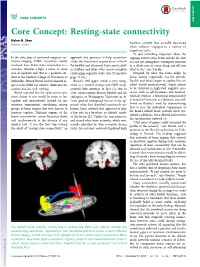
Core Concept: Resting-State Connectivity Helen H
CORE CONCEPTS CORE CONCEPTS Core Concept: Resting-state connectivity Helen H. Shen baseline activity that actually decreased Science Writer when subjects engaged in a variety of cognitive tasks. “It said something important about the In the early days of functional magnetic res- approach that promises to help researchers ongoing activity of the brain, and the fact that onance imaging (fMRI), researchers mostly study the functional organization of both it is not just sitting there waiting for someone analyzed how brain areas responded to a the healthy and abnormal brain, particularly in a white coat to come along and tell you stimulus, whether a light, a noise, or some in children and others who cannot complete what to do,” says Raichle. sort of cognitive task. But as a graduate stu- challenging cognitive tasks. (See Perspective Intrigued by what the brain might be dent at the Medical College of Wisconsin in page 14105.) doing during supposedly inactive periods, Milwaukee, Bharat Biswal had an unusual re- Biswal’s 1995 paper, which is now recog- Raichle and others began to explore this so- quest of his fMRI test subjects: climb into the nized as a seminal resting-state fMRI study, called “default mode network,” which seemed scanner and do, well, nothing. received little attention at first (1). But in to be involved in high-level cognitive pro- Biswal expected that the spontaneous neu- 2001, neuroscientist Marcus Raichle and his cesses, such as self-awareness and memory. ronal chatter at rest would be more or less colleagues at Washington University in St. Michael Greicius, a behavioral neuroscientist random and unstructured. -

The Surprising Role of the Default Mode Network T
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.18.101758; this version posted May 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The Surprising Role of the Default Mode Network T. Brandman1*, R. Malach1, E. Simony1,2. 1Department of Neurobiology and the Azrieli National Institute for Human Brain Imaging and Research, Weizmann Institute of Science. 2Faculty of Engineering, Holon Institute of Technology. *Correspondence to: [email protected] Abstract: The default mode network (DMN) is a group of high-order brain regions recently implicated in processing external naturalistic events, yet it remains unclear what cognitive function it serves. Here we identified the cognitive states predictive of DMN fMRI coactivation. Particularly, we developed a state-fluctuation pattern analysis, matching network coactivations across a short movie with retrospective behavioral sampling of movie events. Network coactivation was selectively correlated with the state of surprise across movie events, compared to all other cognitive states (e.g. emotion, vividness). The effect was exhibited in the DMN, but not dorsal attention or visual networks. Furthermore, surprise was found to mediate DMN coactivations with hippocampus and nucleus accumbens. These unexpected findings point to the DMN as a major hub in high-level prediction-error representations. The default mode network (DMN) is a group of high-order brain regions, so-called for its decreased activation during tasks of high attentional demand, relative to the high baseline activation of the DMN at rest (1-3). -

Glutamate Connectivity Associations Converge Upon the Salience Network in Schizophrenia and Healthy Controls Robert A
McCutcheon et al. Translational Psychiatry (2021) 11:322 https://doi.org/10.1038/s41398-021-01455-y Translational Psychiatry ARTICLE Open Access Glutamate connectivity associations converge upon the salience network in schizophrenia and healthy controls Robert A. McCutcheon 1,2,3,4, Toby Pillinger 1,2,3,4, Maria Rogdaki 1,2,3,4, Juan Bustillo5,6 and Oliver D. Howes1,2,3,4 Abstract Alterations in cortical inter-areal functional connectivity, and aberrant glutamatergic signalling are implicated in the pathophysiology of schizophrenia but the relationship between the two is unclear. We used multimodal imaging to identify areas of convergence between the two systems. Two separate cohorts were examined, comprising 195 participants in total. All participants received resting state functional MRI to characterise functional brain networks and proton magnetic resonance spectroscopy (1H-MRS) to measure glutamate concentrations in the frontal cortex. Study A investigated the relationship between frontal cortex glutamate concentrations and network connectivity in individuals with schizophrenia and healthy controls. Study B also used 1H-MRS, and scanned individuals with schizophrenia and healthy controls before and after a challenge with the glutamatergic modulator riluzole, to investigate the relationship between changes in glutamate concentrations and changes in network connectivity. In both studies the network based statistic was used to probe associations between glutamate and connectivity, and glutamate associated networks were then characterised in terms of their overlap with canonical functional networks. Study A involved 76 individuals with schizophrenia and 82 controls, and identified a functional network negatively associated with glutamate concentrations that was concentrated within the salience network (p < 0.05) and did not 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; differ significantly between patients and controls (p > 0.85). -

The Salience Network Contributes to an Individual's Fluid Reasoning Capacity
G Model BBR-7508; No. of Pages 7 ARTICLE IN PRESS Behavioural Brain Research xxx (2012) xxx–xxx Contents lists available at SciVerse ScienceDirect Behavioural Brain Research j ournal homepage: www.elsevier.com/locate/bbr Research report The salience network contributes to an individual’s fluid reasoning capacity a a a b a,∗ a,c,∗∗ Zhina Yuan , Wen Qin , Dawei Wang , Tianzi Jiang , Yunting Zhang , Chunshui Yu a Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China b LIAMA Center for Computational Medicine, National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences, Beijing, China c School of Medical Imaging, Tianjin Medical University, Tianjin, China a r t i c l e i n f o a b s t r a c t Article history: Fluid reasoning is the ability to think flexibly and logically, analyze novel problems and identify the rela- Received 18 October 2011 tionships that underpin these problems independent of acquired knowledge. Although many functional Received in revised form 14 January 2012 imaging studies have investigated brain activation during fluid reasoning tasks, the neural correlates of Accepted 17 January 2012 fluid reasoning remain elusive. In the present study, we aimed to uncover the neural correlates of fluid Available online xxx reasoning by analyzing correlations between Raven’s Standard Progressive Matrices (RSPM), an effective measure of fluid reasoning, and measures of regional gray matter volume (GMV) and regional homogene- Keywords: ity (ReHo) in a voxel-wise manner throughout the whole brain in 297 healthy young adults. The most Fluid reasoning important finding was that RSPM scores were positively correlated with both GMV and ReHo values in Raven’s Progressive Matrices brain areas that belong to the salience network, including the dorsal anterior cingulate cortex and the Gray matter volume Voxel-based morphometry fronto-insular cortex. -

Neuroscientific Perspectives
Neuroscientific Perspectives © Mindful Schools | All Rights Reserved | mindfulschools.org Mindfulness Meditation & the Default Mode Network (DMN) In an important study, Malia Mason of Columbia University wrote,1 What does the mind do in the absence of external demands for thought? Is it essentially blank, springing into action only when some task requires attention? Everyday experience challenges this account of mental life. In the absence of a task that requires deliberative processing, the mind generally tends to wander, flitting from one thought to the next with fluidity and ease. Given the ubiquitous nature of this phenomenon, it has been suggested that mind-wandering constitutes a psychological baseline from which people depart when attention is required elsewhere and to which they return when tasks no longer require conscious supervision. She and her colleagues proceeded to demonstrate that mind wandering is associated with increased activation in the ‘default mode network’ – brain regions including the posterior cingulate and the medial prefrontal cortex. The researchers were able to correlate increased activity in these brain regions with individuals’ reports regarding their own mind wandering. The DMN is a clear target for meditative practice. Mindfulness is often considered an attentional training. Preliminary but intriguing data suggest that mindfulness practice may target the DMN and change its functioning. Judson Brewer and his colleagues did research in 2011 on this subject. They wrote:2 Mind-wandering is not only a common activity present in roughly 50% of our awake life, but is also associated with lower levels of happiness. Moreover, mind- wandering is known to correlate with neural activity in a network of brain areas that support self-referential processing, known as the default mode network (DMN). -

Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: a Central Pathway in Psychiatric Disease and Treatment
REVIEW published: 27 December 2016 doi: 10.3389/fnsys.2016.00104 Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment Sarah K. Peters 1, Katharine Dunlop 1 and Jonathan Downar 1,2,3,4* 1Institute of Medical Science, University of Toronto, Toronto, ON, Canada, 2Krembil Research Institute, University Health Network, Toronto, ON, Canada, 3Department of Psychiatry, University of Toronto, Toronto, ON, Canada, 4MRI-Guided rTMS Clinic, University Health Network, Toronto, ON, Canada The salience network (SN) plays a central role in cognitive control by integrating sensory input to guide attention, attend to motivationally salient stimuli and recruit appropriate functional brain-behavior networks to modulate behavior. Mounting evidence suggests that disturbances in SN function underlie abnormalities in cognitive control and may be a common etiology underlying many psychiatric disorders. Such functional and anatomical abnormalities have been recently apparent in studies and meta-analyses of psychiatric illness using functional magnetic resonance imaging (fMRI) and voxel- based morphometry (VBM). Of particular importance, abnormal structure and function in major cortical nodes of the SN, the dorsal anterior cingulate cortex (dACC) and anterior insula (AI), have been observed as a common neurobiological substrate across a broad spectrum of psychiatric disorders. In addition to cortical nodes of the SN, the network’s associated subcortical structures, including the dorsal striatum, mediodorsal thalamus and dopaminergic brainstem nuclei, comprise a discrete regulatory loop circuit. Edited by: The SN’s cortico-striato-thalamo-cortical loop increasingly appears to be central to Avishek Adhikari, mechanisms of cognitive control, as well as to a broad spectrum of psychiatric illnesses Stanford University, USA and their available treatments. -

Changes in Extra-Striatal Functional Connectivity in Patients with Schizophrenia in a Psychotic Episode
The British Journal of Psychiatry (2017) 210, 75–82. doi: 10.1192/bjp.bp.114.151928 Changes in extra-striatal functional connectivity in patients with schizophrenia in a psychotic episode Henning Peters, Valentin Riedl, Andrei Manoliu, Martin Scherr, Dirk Schwertho¨ ffer, Claus Zimmer, Hans Fo¨ rstl, Josef Ba¨ uml, Christian Sorg* and Kathrin Koch* Background In patients with schizophrenia in a psychotic episode, with right anterior insula and dorsal prefrontal cortex, intra-striatal intrinsic connectivity is increased in the putamen the ventral striatum with left anterior insula. Decreased but not ventral striatum. Furthermore, multimodal changes functional connectivity between putamen and right have been observed in the anterior insula that interact anterior insula was specifically associated with patients’ extensively with the putamen. hallucinations. Functional connectivity distinctiveness was impaired only for the putamen. Aims We hypothesised that during psychosis, putamen extra- Conclusions striatal functional connectivity is altered with both the Results indicate aberrant extra-striatal connectivity during anterior insula and areas normally connected with the psychosis and a relationship between reduced putamen–right ventral striatum (i.e. altered functional connectivity anterior insula connectivity and hallucinations. Data distinctiveness of putamen and ventral striatum). suggest that altered intrinsic connectivity links striatal and Method insular pathophysiology in psychosis. We acquired resting-state functional magnetic resonance -

Salience Network-Midbrain Dysconnectivity and Blunted Reward Signals in Schizophrenia
öÌ·¬´» п¹» •¸±©·²¹ º«´´ ß«¬¸±® ¿²¼ ß¼¼®»•• Ü»¬¿·´• Salience network-midbrain dysconnectivity and blunted reward signals in schizophrenia Victoria B. Gradina*, Gordon Waiterb, Akira O'Connorc, Liana Romaniukd, Catriona Sticklee, Keith Matthewsa, Jeremy Halld, J. Douglas Steelea a Medical Research Institute, University of Dundee, UK b Biomedical Imaging Centre, University of Aberdeen, UK c Department of Psychology, University of St Andrews, UK d Division of Psychiatry, University of Edinburgh, UK; e Royal Cornhill Hospital, Aberdeen, UK Abstract words: 196 < 200 *Corresponding author: Dr. Victoria Gradin Division of Neuroscience (Mailbox 5) Medical Research Institute Dundee University Ninewells Hospital & Medical School Dundee DD1 9SY Email: [email protected]; [email protected] Tel: *44-1382-496-233 Fax *44-1382-633-865 öλª·•»¼ Ó¿²«•½®·°¬ Abstract Theories of schizophrenia propose that abnormal functioning of the neural reward system is linked to negative and psychotic symptoms, by disruption of reward processing and promotion of context-independent false associations. Recently it has been argued that an insula-anterior cingulate cortex (ACC) salience network system enables switching of brain states from the default mode to a task-related activity mode. Abnormal interaction between the insula-ACC system and reward processing regions may help explain abnormal reinforcer processing and symptoms. Here we use fMRI to assess the neural correlates of reward processing in schizophrenia. Furthermore we investigated functional connectivity between the dopaminergic midbrain, a key region for the processing of reinforcers, and other brain regions. In response to rewards, controls activated task related regions (striatum, amygdala/hippocampus and midbrain) and the insula-ACC salience network. Patients similarly activated the insula-ACC salience network system but failed to activate task related regions. -

The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: a Review
The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Mohan, Akansha, Aaron J. Roberto, Abhishek Mohan, Aileen Lorenzo, Kathryn Jones, Martin J. Carney, Luis Liogier-Weyback, Soonjo Hwang, and Kyle A.B. Lapidus. 2016. “The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review.” The Yale Journal of Biology and Medicine 89 (1): 49-57. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:26318604 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA YALE JOURNAL OF BIOLOGY AND MEDICINE 89 (2016), pp.49-57. REviEW The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review Akansha Mohan, BA a; Aaron J. Roberto, MD b*; Abhishek Mohan, BS c; Aileen Lorenzo, MD d; Kathryn Jones, MD, PhD b; Martin J. Carney, BS e; Luis Liogier-Weyback, MD f; Soonjo Hwang, MD g; and Kyle A.B. Lapidus, MD, PhD h aBaylor College of Medicine, Houston, Texas; bClinical fellow, Child and Adolescent Psychiatry, Boston Children’s Hospital, Harvard Medical School, Boston, MA; cOld Dominion University, Norfolk, VA; dResident physician, Adult Psychiatry, West- chester Medical Center, -

Neuroimage: Clinical 15 (2017) 513–524
NeuroImage: Clinical 15 (2017) 513–524 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Disruption to control network function correlates with altered dynamic MARK connectivity in the wider autism spectrum ⁎ N. de Lacya,b, D. Dohertyb,c, B.H. Kingd, S. Rachakondae, V.D. Calhoune,f, a Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA 98195, USA b Seattle Children's Research Institute, Center for Integrative Brain Research, Seattle, WA 98105, USA c Department of Pediatrics, Divisions of Developmental and Genetic Medicine, University of Washington, Seattle, WA 98195, USA d Department of Psychiatry, University of California San Francisco, San Francisco, CA 94143, USA e The Mind Research Network & LBERI, Albuquerque, NM 87106, USA f Department of Electrical and Computer Engineering, University of New Mexico, Albuquerque, NM 87131, USA ARTICLE INFO ABSTRACT Keywords: Autism is a common developmental condition with a wide, variable range of co-occurring neuropsychiatric Autism symptoms. Contrasting with most extant studies, we explored whole-brain functional organization at multiple Functional MRI levels simultaneously in a large subject group reflecting autism's clinical diversity, and present the first network- Dynamic connectivity based analysis of transient brain states, or dynamic connectivity, in autism. Disruption to inter-network and inter- Control networks system connectivity, rather than within individual networks, predominated. We identified coupling disruption in Task-positive the anterior-posterior default mode axis, and among specific control networks specialized for task start cues and Default-mode the maintenance of domain-independent task positive status, specifically between the right fronto-parietal and cingulo-opercular networks and default mode network subsystems. -

Development of Thalamocortical Connectivity During Infancy and Its Cognitive Correlations
The Journal of Neuroscience, July 2, 2014 • 34(27):9067–9075 • 9067 Development/Plasticity/Repair Development of Thalamocortical Connectivity during Infancy and Its Cognitive Correlations Sarael Alcauter,1 Weili Lin,1 X J. Keith Smith,2 Sarah J. Short,3 Barbara D. Goldman,4 J. Steven Reznick,4 John H. Gilmore,3 and Wei Gao1 1Department of Radiology and Biomedical Research Imaging Center, 2Department of Radiology, 3Department of Psychiatry, and 4Frank Porter Graham Child Development Institute and Department of Psychology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599 Although commonly viewed as a sensory information relay center, the thalamus has been increasingly recognized as an essential node in various higher-order cognitive circuits, and the underlying thalamocortical interaction mechanism has attracted increasing scientific interest. However, the development of thalamocortical connections and how such development relates to cognitive processes during the earliest stages of life remain largely unknown. Leveraging a large human pediatric sample (N ϭ 143) with longitudinal resting-state fMRI scans and cognitive data collected during the first 2 years of life, we aimed to characterize the age-dependent development of thalamo- cortical connectivity patterns by examining the functional relationship between the thalamus and nine cortical functional networks and determine the correlation between thalamocortical connectivity and cognitive performance at ages 1 and 2 years. Our results revealed that the thalamus–sensorimotor