Analysis of Groundwater Quality Using Water Quality Index: a Case Study of Simhadripuram Mandal, YSR Kadapa District, Andhra Pradesh
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
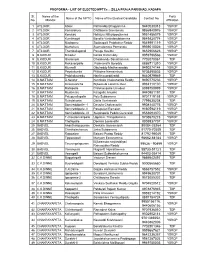
Sl. No. Name of the Mandal Name of the MPTC Name of the Elected
PROFORMA - LIST OF ELECTED MPTCs :: ZILLA PRAJA PARISHAD, KADAPA Sl. Name of the Party Name of the MPTC Name of the Elected Candidate Contact No No. Mandal Affiliation 1 ATLOOR Atloor Pothireddy Bhagyamma 9440030313 YSRCP 2 ATLOOR Kamalakuru Chittiboina Sreeramulu 9866940976 YSRCP 3 ATLOOR Konduru Nelaturu Nithyapoojamma 9951486079 YSRCP 4 ATLOOR Kumbhagiri Bandila Venkatasubbamma 9849828774 YSRCP 5 ATLOOR Madapuru Sodinapalli Prabhakar Reddy 9849991119 YSRCP 6 ATLOOR Muthukuru Syamalamma Ponnavolu 9959010026 YSRCP 7 ATLOOR Thamballagondi Perugu Savithri 9652906623 YSRCP 8 B.KODUR B.Kodur Konda Ramireddy 9959796566 YSRCP 9 B.KODUR Itharampet Chatakondu Sanathamma 7702070061 TDP 10 B.KODUR Mekavaripalle Padmavathi Boreddy 8886711310 YSRCP 11 B.KODUR Munnelli Obulreddy Madhavareddy 9490274144 YSRCP 12 B.KODUR Payalakuntla Pilliboina Narasimhulu 9703490503 YSRCP 13 B.KODUR Prabhalaveedu Neelima peddireddi 9440979949 TDP 14 B.MATTAM D.Nelatur Kunchala Vivekananda Reddy 9490770236 YSRCP 15 B.MATTAM Dirasavancha Bijivemula Lakshmi Devi 9963110130 YSRCP 16 B.MATTAM Mallepalle Chilekampalle Umadevi 8099750999 YSRCP 17 B.MATTAM Mudamala Kalagotla Anusha 9440981191 TDP 18 B.MATTAM Palugurallapalle Polu Subbamma 9701719158 YSRCP 19 B.MATTAM Rekalakunta Obilla Venkataiah 7799630208 TDP 20 B.MATTAM Somireddipalle -1 Devarla Chakravarthi 9908140775 YSRCP 21 B.MATTAM Somireddipalle -II Pasupuleti Ramaiah 9160594119 YSRCP 22 B.MATTAM Somireddipalle -III Sugalapalle Pedda Guravaiah 9553693370 YSRCP 23 B.MATTAM T.choudarivaripalle Uppaluri. Thirupalamma -

Jurisdiction of Nellore Central Excise and Service Tax Commissionerate
Page 1 Annexure-A to Trade Notice No: 1 /2014 dated 07/10/2014 of Visakhapatnam Zone Jurisdiction of Nellore Central Excise and Service Tax Commissionerate Commissionerate Jurisdiction In the Revenue Districts of Dr.Y.S.Rajasekhara Reddy Kadapa District, Sri Potti Sriramulu NELLORE Nellore District and Prakasam District in the State of Andhra Pradesh Sl. Name of the Jurisdiction of the Division Name of the Jurisdiction of the Range No. Division Range The revenue Mandals of Kadapa, Brahmamgari matam, Chintakommadinne, Chennur, Khazipet, Badvel, Porumamilla, KADAPA Kalasapadu, Kasinayana, Sidhout, Gopavaram, Atluru, Ontimitta, Valluru, B.Koduru and Pendlimarri of Kadapa District The revenue Mandals of Proddatur, Rayachoti, T.Sundupalli, Lakkireddypalli, Ramapuram, Chakrayapet, Galivedu, Peddamudium, PRODDATUR Vempalli, Duvvuru, Sambepalli, Vemula, Jammalamadugu, Mylavaram, Chapadu, Mydukur, Kamalapuram, Veerapunayunipalli, In the Revenue District of Veeraballi, Rajupalem and Chinnamandem of Kadapa District Kadapa Division Dr.Y.S.Rajasekhara Reddy 1 (Central Excise Kadapa in the State of Andhra The revenue Mandals of Pulivendula, Lingala, Tonduru, Muddanuru, & Service Tax) CHILAMKUR Pradesh Simhadripuram, Kondapuram of Kadapa District. The revenue Mandals of Nandalur, Rajampet, Chitvel, Kodur, NANDALUR Pullampeta, Obulavaripalli and Penagaluru of Kadapa District. YERRAGUNTLA The entire Yerraguntla revenue Mandal of Kadapa District SERVICE TAX Entire Dr.Y.S.Rajasekhara Reddy Kadapa Revenue District RANGE Page 2 Annexure-A to Trade Notice No: -
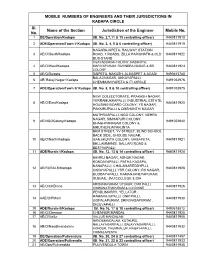
Sl. No. Name of the Section Jurisdiction of the Engineer Mobile
MOBILE NUMBERS OF ENGINEERS AND THEIR JURISDICTIONS IN KADAPA CIRCLE Sl. Name of the Section Jurisdiction of the Engineer Mobile No. No. 1 DE/Operation/Kadapa (Sl. No. 2,7,11 & 15 controlling officer) 9440811918 2 ADE/Operation/Town I/ Kadapa (Sl. No. 3, 4, 5 & 6 controlling officer) 9440811919 NAGARAJUPETA, RAILWAY STATION 3 AE/O/South/Kadapa ROAD, 7 ROADS, ZILLA PARISHATH & OLD 9440811922 BUS-STAND VIJAYADURGA COLONY, NABIKOTA, 4 AE/O/West/Kadapa MARIYAPURAM, RAVINDRA NAGAR & SBI 9440811923 COLONY 5 AE/O/Saipeta SAIPETA, NAKASH, ALMASPET & AGADI 9490615780 BALAJINAGAR, AKKAYAPALLI, 6 AE/ Balaji Nagar/ Kadapa 9491052976 CHEMMUMIYAPETA & ITI CIRCLE 7 ADE/Operation/Town II/ Kadapa (Sl. No. 8, 9 & 10 controlling officer) 9491052975 NEW COLLECTORATE, PRAKASH NAGAR, YERRAMUKKAPALLI, INDUSTRIAL ESTATE, 8 AE/O/East/Kadapa 9440811920 HOUSING BOARD COLONY, YS NAGAR, PAKKIRUPALLI & OMSHANTHI NAGAR MUTHRASPALLI, NGO COLONY, NEHRU NAGAR, SIMHAPURI COLONY, 9 AE/NGOColony/Kadapa 9491052940 BHAGHYANAGAR COLONY & MRUTHUNJAYAKUNTA BKM STREET, YV STREET, BLIND SCHOOL BACK SIDE, GHOUSE NAGAR, 10 AE/O/North/Kadapa GANJIKUNTA COLONY, MASAPETA, 9440811921 BELLAMMANDI, BALLARI ROAD & BESTHAPALLI 11 ADE/Rurals-I/Kadapa (Sl. No. 12, 13 & 14 controlling officer) 9440811924 NEHRU NAGAR, ASHOK NAGAR, KONDAYAPALLI, PATHA KADAPA, NANAPALLI, CHALAMAREDDYPLLI, 12 AE/RURALS/Kadapa 9440811928 UKKAYAPALLI, YSR COLONY, RK NAGAR, BUDDAYAPALLI, RAMANJANEYAPURAM, SUBJAIL, JMJ COLLEGE & IDA KRISHNAPURAM, UTUKUR, CMR PALLI, 13 AE/O/CKDinne 9440811926 VISWANATHAPURAM & C.K DINNE PENDLIMARRY, YELLATUR , RAMARAJUPALLI, CNR PALLI, 14 AAE/O/PMarri 9440811930 GOPALAPURAM, SRINIVASAPURAM , DIGUVAPALLI 15 ADE/Rurals-II/Kadapa (Sl. No.16, 17 & 18 controlling officer) 9490618812 16 AE/O/Chennur CHENNUR MANDAL 9440811925 17 AE/O/Vallur VALLUR MANDALAM 9440811955 NANDIMANDALAM, KOTHURU, NALLAYAGARIPALLI, BALAYYAGARIPALLI, 18 AE/O/Nandimandalam 9440811932 KONDUR, THUMMALUR, NAGAYAPALLI, CHIMALAPENTA 19 DE/Operation/Pulivendula (Sl. -

Name of the District:Kadapa S.No Bank Mandal Village HH
SLBC OF AP CONVENOR:ANDHRA BANK FIP-- ABOE 2000 POPULATION VILLAGES Name of the District:Kadapa S.No Bank Mandal Village HH Population 1 Andhra Bank Jammalamadugu Goriganur 569 3005 2 Atlur Kamalakuru 667 2929 3 Atlur Madapur 558 2269 4 C K Dinne Utukur 1163 5320 5 C K Dinne Viswanathapuram 537 2511 6 C K Dinne Mamillapalle 1668 7330 7 C K Dinne Ippapenta 703 3122 8 T.Sundupalle Polimerapalle 1022 4320 9 Rajampet Poli 1119 5159 10 Pullampet Ananthaiahgaripalli 818 3499 11 APGB Mylavaram Chinna Komerla 815 3434 12 Mylavaram Thorrivemula 599 2467 13 Mylavaram Veparala 1627 6616 14 Mylavaram Dommara Nandyala 1813 7771 15 Peddamudium Gundlakunta 451 3385 16 Peddamudium Boditippanapadu 392 2055 17 Peddamudium Suddapalle 513 2243 18 Peddamudium Jangalapalle 706 3026 19 Rajupalem Arakatavemula 633 2569 20 Rajupalem Parlapadu 577 2399 21 Rajupalem Velavali 905 3878 22 Duvvur Idamadaka 714 2854 23 Mydukur Settivaripalle 1551 6813 24 Brahmamgarimattam Mallepalle 852 3675 25 Brahmamgarimattam Palagurallapalle 857 3725 26 Brahmamgarimattam Somireddipalle 1758 8149 27 Brahmamgarimattam Jangamrajupalle 644 2909 28 SAKN Katheragandla 1624 7006 29 SAKN Savisettipalle 708 3038 30 Kalasapadu Kothakota 606 2985 31 Kalasapadu Sankavaram 1945 8276 32 Porumamilla Ganugapenta 485 2364 33 B.Kodur Munelli 1394 6442 34 Gopavaram N Ramapuram 567 2432 35 Gopavaram S Ramapuram 693 2987 36 Khajipet Midthuru 616 2635 37 Khajipet Chemullapalle 997 4236 38 Chapadu Alladupalle 1085 4178 39 Chapadu Ananthapuram 1162 4903 40 Chapadu Vedurur 879 3654 41 Proddatur Gopavaram -

1 YSR Kadapa LA8021779931 APPALAROUTHU RAVINDRA Male 7993180217 600 600 100 18/01/1999 10 10 100 1 2 YSR Kadapa LA8382888977
Lab Ateendant Applicants Rank Details S.No District Name Application Applicant Name Gender Mobile SSC SSC SSC % Date Of Birth SSC SSC SSC Rank Number Number Total Obtai General General General Mark ned Science Science s Mark Science Obtaine Marks s Total d Marks Percent 1 YSR Kadapa LA8021779931 APPALAROUTHU RAVINDRA Male 7993180217 600 600 100 18/01/1999 Marks10 10 age100 1 2 YSR Kadapa LA8382888977 RAYACHOTY MEGHANA FeMale 8897783828 600 600 100 18/06/2000 100 100 100 2 3 YSR Kadapa LA8754689778 THOTA RAMA SRUJAN Male 8977887546 600 590 98.33 12/06/1999 100 100 100 3 4 YSR Kadapa LA4415277995 MITTA SIVAPARVATHI FeMale 7799544152 600 590 98.33 20/06/2000 100 100 100 4 5 YSR Kadapa LA1178099490 SALINDRA POORNA FeMale 9949011780 600 590 98.33 26/10/2000 100 100 100 5 6 YSR Kadapa LA5985680198 BANTROTHU VYSHNAVI FeMale 8019859856 600 590 98.33 20/06/2001 100 90 90 6 7 YSR Kadapa LA5385091773 GUDAMCHERLA MOHAMMAD Male 9177353850 600 590 98.33 06/09/2001 100 90 90 7 AKRAM 8 YSR Kadapa LA5830399665 JANJALA SATEESHA FeMale 9966558303 600 590 98.33 03/04/2002 100 100 100 8 9 YSR Kadapa LA3191391334 DUDEKULA KULLAI SHAREEF Male 9133431913 600 588 98 07/05/2002 100 100 100 9 10 YSR Kadapa LA3919899598 NEELAM SANDYA FeMale 9959839198 600 585 97.5 15/01/2001 100 90 90 10 11 YSR Kadapa LA8065062816 POOLA MAHAMMADSHAHID Male 6281680650 600 580 96.67 16/05/1997 100 100 100 11 HUSSAIN 12 YSR Kadapa LA7026091823 Sirigireddy jagan mohan Male 9182370260 600 580 96.67 21/08/1997 100 100 100 12 reddy 13 YSR Kadapa LA2226879818 MADAKA SIVA KUMAR Male 7981822268 -
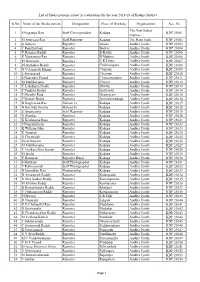
S.No Name of the Media Person Designation Place of Working Organization Acc
List of Media persons issued Accreditations for the year 2018-19 of Kadapa District S.No Name of the Media person Designation Place of Working Organization Acc. No The New Indian 1 S Nagaraja Rao Staff Correspondent Kadapa KDP 23001 Express 2 M Srinivasa Rao Staff Reporter Kadapa The Hans India KDP 23002 3 A Sanjeev Reporter Atloor Andhra Jyothi KDP 23003 4 T Ramthirtham Reporter Badvel Andhra Jyothi KDP 23004 5 V Ramana Reddy Reporter B Kodur Andhra Jyothi KDP 23005 6 K Nageswara Rao Reporter B Mattam Andhra Jyothi KDP 23006 7 M Narayana Reporter C.K.Dinne Andhra Jyothi KDP 23007 8 M Sudhakar Reddy Reporter Chakrayapeta Andhra Jyothi KDP 23008 9 N V Sampath Kumar Reporter Chapadu Andhra Jyothi KDP 23009 10 L Sivaprasad Reporter Chennur Andhra Jyothi KDP 23010 11 S Nagendra Prasad Reporter Chinnamandem Andhra Jyothi KDP 23011 12 M Mallikarjuna Reporter Chitvel Andhra Jyothi KDP 23012 13 Y Lakshman Naidu Reporter Duvvur Andhra Jyothi KDP 23013 14 P Venkata Reddy Reporter Galiveedu Andhra Jyothi KDP 23014 15 P Maruthi Raju Reporter Gopavaram Andhra Jyothi KDP 23015 16 P Mastan Basha Reporter Jammalamadugu Andhra Jyothi KDP 23016 17 B Nageswara Rao Edition I/c Kadapa Andhra Jyothi KDP 23017 18 B Govinda Swamy Bureau I/c Kadapa Andhra Jyothi KDP 23018 19 E Anjaneyulu Staff Reporter Kadapa Andhra Jyothi KDP 23019 20 N Shankar Reporter Kadapa Andhra Jyothi KDP 23020 21 K Krishnama Raju Reporter Kadapa Andhra Jyothi KDP 23021 22 P Nagamallaiah Reporter Kadapa Andhra Jyothi KDP 23022 23 K William John Reporter Kadapa Andhra Jyothi KDP 23023 24 -

Anantapur Ysr(Kadapa)
77°0'0"E 77°30'0"E 78°0'0"E 78°30'0"E 79°0'0"E 79°30'0"E X XXX GXuXXXnXXtakal X X XX (! XX X X GEOGRAPHICAL AREA ANANTAPUR AND XX XX X NH67 Gadekallu X X ¤£ !( CA05 CAX(! 06X X CA114 YSR (KADAPA) DISTRICTS X GUNTAKAL GOXOTY XX CA23 !( CA03 X X KALASSPSDU !( Ravuludiki N X X CA113 !( !(X !( " Kanuru YADIKI VIDAPANAKAL !( X X 0 ' CA02 !(Havalige X SRI AVADHUTHAKASINAYANA N 0 X X X " KEY MAP ° CA04 CA07 X X 0 5 Palturu X BOMMANAHAL !( X CA105 (! ' 1 X X NH544D CA112 0 VAJRAKARUR X PEDDAVADUGUR ¤£ ° X X CA01 (! CA08 X PEDDAMUDIUN CA106 ± 5 Nerametla !( Uravakonda X Pamidi XXX !( PORUMAMILLA 1 (! X CA24 CA22 X Pagidala !( D.HIREHAL PAMIDI CA103 !(!( RAJUPALEM X (! !( TADPATRI X !( Somapur!(am CA10 PEDDAPAPPUR MYLAVARAM !( C!( !(A108 !( !(Mutssukota Vengalayapalle CA20 X !( X Sanagal Gudur Jammalamadugu !( !( Mudireddipalle CA111 URAVAKONDA CA21 !( (! DUVVU!( R !( !( !( !( Muradi CA11 !( Pottipadu !( !( !( GUDIBANDA X !( Duvvuru SINGANAMALA !( !( !( CA109 B.KODUR X CA!( 102 Huchchangidurga KANEKAL !( X !( !( CA10!( 4 Gopavaram !( CA09 !( !( X !( !( CA110 !( CA25 !( !( S.MYDUKUR CA19 KONDAPURAM !( !( Madur X !( !( !( !( !( !( !( X JAMMALAMDUXGX(!U !( KUDAIR!( X Ellanuru ObalapuraXmX !( X Annavaram BRAHMAMGARIMATTAM CA12 !( BUKKARAYA SAMUDRAMPUTLUR !( X!( Peddandlur Mydukuru !( (! (! Kuderu !( X !( !( (! XX X !( Badvel RAX YADURG CA15 X Mangapatnam !( Virapalle CA28 X XXX !(!( CA100 X (! X X XXX!( X X .! !( X BELUGUPPA X !( CA18 NARPALA CA26 CA101 YERRAGUNTLA !( !( X CA97 Chi!(lamkuru !( CA13 Simhadripuram !( Golla CA17 X !( !( X Niduzuvvi CA99 -

Kadapa Kadapa and Pulivendula Courts 2
1 Territorial Jurisdiction of Criminal Courts S.No. Name of the Court Name of the Police Stations 1. Principal District and Sessions Judge’s Police Stations covered by Court, Kadapa Kadapa and Pulivendula Courts 2. I Additional District and Sessions Judge’s Police Stations covered by Court, Kadapa Kadapa and Pulivendula Courts 3. II Additional District and Sessions Police Stations covered by Judge’s Court, Kadapa at Proddatur Proddatur, Mydukur, Jammalamadugu and Kamalapuram Courts 4. III Additional District and Sessions Police Stations covered by Judge’s Court, Rajampet Rajampet, Nandalur, Railway Kodur Courts 5. IV Additional District and Sessions Police Stations covered by Judge’s Court, Kadapa Kadapa and Pulivendula Courts 6. V Additional District and Sessions Police Stations covered by Judge’s Court, Rayachoty Rayachoty and Lakkireddipalli Courts 7. Judge, Family Court-Cum-Additional Police Stations covered by District Court, Kadapa Kadapa and Pulivendula Courts 8. Senior Civil Judge, Kadapa Police Stations covered by Kadapa and Pulivendula Courts and Khajipet P.S. 9. Addl. Senior Civil Judge, Kadapa Police Stations covered by Kadapa and Pulivendula Courts and Khajipet P.S. 10. Senior Civil Judge, Proddatur Police Stations covered by Proddatur, Mydukur, Jammalamadugu and Kamalapuram Courts except Khajipet P.S. 11. Senior Civil Judge, Rayachoty Police Stations covered by Rayachoty and Lakkireddipalli Courts 12. Senior Civil Judge, Rajampet Police Stations covered by Rajampet, Nandalur and Railway Kodur Courts 13. Additional Senior Civil Judge (FTC), Police Stations covered by Rajampet at Badvel Badvel and Sidhout Courts 14. I Addl. Junior Civil Judge, Kadapa Kadapa I Town P.S Kadapa II Town P.S Women P.S. -

Data Base of Daattc ,Ysr Kadapa District
DATA BASE OF DAATTC ,YSR KADAPA DISTRICT Land utilization pattern: Total geographical area - 15,35,900 ha Gross cultivated area - 5,02,402 ha Net area sown - 4,05,276 ha Rainfed area - 3,12,062 ha Net irrigated area - 1,90,340 ha Area cultivated more than once - 97,126 ha Extent (ha) % Total area Name of the agricultural division Soil type Red soils 1,70,216 42 LR Palli, Rayachoty, Pulivendula, Rajampet, Rly.Kodur divisions. Black 1,98,585 49 Muddanur, Jammalamadugu, soils Proddatur, Pulivendula & Kamalapuram divisions. Sand & 36,475 9 Kadapa, Kamalapuram, Vallur Saline soils Porumamilla, LR Palli, Rayachoty. Irrigation Major, Medium irrigation sources and area irrigated Name of the project Registered Ayacut Actual area irrigated (Ha) Gross Net Kurnool Kadapa Canal 37117 24399 22497 Telugu Ganga Project 64330 1687 1542 Pullivendula Branch 37186 612 612 Canal including Kadapa Branch Canal Buggavanka 5204 429 347 Cheyyuru(Annammayya) 9112 0 0 Lower Sagileru 5212 2189 1259 Upper Sagileru 2144 0 0 Pincha 1528 802 802 Total 161833 30118 27059 Area irrigated under different sources in hectare S.No Sources of 2006-07 2007-08 2008-9 2009-10 2010-11 irrigation 1 Canals 0 0 30571 26047 30118 2 Tanks 0 0 7524 4442 8208 3 Tube wells 0 0 147486 155660 157324 4 Lift irrigation 0 0 554 975 1044 5 Dug wells 0 0 6030 5725 5593 6 Other sources 149809 179598 88 11 213 7 Gross area 149809 17598 192253 192860 202500 irrigated 8 Area irrigated 25497 32163 32574 40568 43247 more than once 9 Net area irrigated 124312 147435 159679 152292 159253 Category wise -
Hand Book of Statistics 2015
HAND BOOK OF STATISTICS Y.S.R. DISTRICT 201 5 CHIEF PLANNING OFFICER Y.S.R. DISTRICT Sri. K.V.Ramana, I.A.S., District Collector & Magistrate, Y.S.R. District P R E F A C E The District Hand Book of Statistics 2015 in its 29th edition contains information of various Departments in the District including data relating to Agriculture, Rainfall and Land Utilization etc., I hope this book will be quite useful to the Public, Planners, Research Scholars, Bankers, Administrators and Non-Governmental Organizations. The continuous and generous co-operation extended by the District Officers in supplying the data for bringing out this publication is specially acknowledged. The Officers and Staff of the Chief Planning Office working in the District have done a commendable job in bringing out this publication. District Administration welcomes the constructive suggestions and additional information for improvement of this Hand Book. DISTRICT COLLECTOR, Y.S.R DISTRICT. OFFICERS AND STAFF ASSOCIATED WITH THE PUBLICATION Sl.No Name of the Officer Designation 1 Sri. V. THIPPESWAMY CHIEF PLANNING OFFICER 2 Sri. K. DEVANAND ASSISTANT DIRECTOR 3 Sri. N.RAMAMURTHY NAIDU STATISTICAL OFFICER 4 Sri. R. PRABHAKAR RAO DY. STATISTICAL OFFICER 5 Smt P. SARASWATHI DEVI TYPIST I N D E X TABLE CONTENTS PAGE Nos. NO. A SALIENT FEATURES OF THE DISTRICT : NARRATIVE PART I – XXIII B COMPARISION OF THE DISTRICT WITH THE STATE 2014-15 XXIV – XXIX C ADMINISTRATIVE DIVISIONS IN THE DISTRICT – 2014-2015 XXX C (1) MUNICIPAL INFORMATION IN THE DISTRICT - 2014-15 XXXI D PUBLIC REPRESENTATIVES / NON-OFFICIALS XXXII – XXXVII PROFILE OF PARLIAMENTARY CONSTITUENCY / ASSEMBLY E XXXVIII – XXXX CONSTITUENCY 1 - POPULATION 1.1 VARIATION IN POPULATION, 1901 TO 2011 1-1 1.2 POPULATION STATISTICS, SUMMERY 2001 AND 2011 2-2 MANDAL WISE NO. -

Irrigation Profile of Ysr Kadapa District
10/31/2018 District Irrigation Profiles IRRIGATION PROFILE OF YSR KADAPA DISTRICT **Click here for Ayacut Map INTRODUCTION The Kadapa district was formed in 1808 during British rule with head quarters at Kadapa. There are 51 Mandals in the District. The population of the District is 28,84,524 persons as per Census 2011. The District is bounded on the North by Kurnool and Prakasam districts, on the South by Chitoor district, on the East by Nellore district and on the West by Anantapur district. The District is rich in mineral resources. The principle minerals available are Lead, Barites, Asbestos, Lime stone, Uranium and Kadapa stone. The District Head quarters is connected with broad gauge railway line from Mumbai to Chennai and Hyderabad to Tirupathi. The area of the district is mainly covered under major irrigation i.e., K.C. Canal, Telugu Ganga Project, GNSS Project and No. of other Medium and Minor irrigation sources. The District is situated with in the Geographical Co-ordination of 13° 43' and 15° 14' of Northern latitude and 77° 55' and 79° 29' of the Eastern longitude. The entire District lies in Pennar river Basin. The Major amount of rain falls due to South-West Monsoon. The District is classified as drought Prone area due to Scanty and erratic rain fall. The normal rainfall is 700 mm. The climate is generally warm in Summer. The important http://irrigationap.cgg.gov.in/wrd/getDistricts 1/61 10/31/2018 District Irrigation Profiles rivers in the District are Penna, Chitravathi, Kundu, Sagileru, Papagni, Bahuda and Cheyyeru The -

The Special Deputy Collector (L.A), PBC/MRC, Kadapa Further Reported
కడప 瀿졍ా ల గెజె籍 KADAPA DISTRICT GAZETTE EXTRA-ORDINARY) ( PUBLISHED BY AUTHORITY Gazette No.22-1 KADAPA 19.03.2020 PROCEEDINGS OF THE SPECIAL COLLECTOR (L.A),GNSS., KADAPA PRESENT::SRI D.SATISH CHANDRA, M.Com., L.L.B., Ref.No.B/160/2018. Dated: 19.03.2020 Notification by the Government GOVERNMENT OF ANDHRA PRADESH Order of Extension of Time U/s 25 of the RFCTLARR Act, 2013 The Special Deputy Collector (L.A), PBC/MRC, Kadapa further reported that, basing on the requisition of the Executive Engineer, PBC Division, Pulivendula, for acquisition of lands for 2L Minor of Balapanur Distributory under Pulivendula Branch Canal, the Special Deputy Collector (L.A), PBC/MRC,Kadapa has submitted Preliminary Notification proposal in the reference 2nd cited for approval and the same was approved by the Special Collector(L.A), G.N.S.S., Kadapa vide this office references 3rd cited. Further, the Special Deputy Collector (L.A), PBC/MRC, Kadapa has submitted the details of Survey and Census of affected families as required U/s 16 (1) & 16(2) of the RFCT LARR Act, 2013 for approval and the same was approved by the Joint Collector & Project Administrator, Kadapa vide this office Ref. No. B/160/2018, Dt: 30.08.2018. Thereupon the Special Deputy Collector (L.A), PBC/MRC, Kadapa has submitted the „NIL‟ Final R& R scheme proposals U/s 17 of the RFCT LARR Act, 2013 for approval and the same was also approved by the District Collector, Kadapa vide this office Ref.No. B/160 /2018, Dt: 18.11.2018.Thereafter the Special Deputy Collector has submitted declaration proposals vide her office Ref No.B/19/2018 Dt: 23.11.2018 and the same was approved by the Special Collector,(LA) GNSS., Kadapa in the reference 9th read above.