Download Articles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Maria Curie-Skłodowska University Botanical Garden in Lublin As a Refuge of the Moths (Lepidoptera: Heterocera) Within the City
Acta Biologica 23/2016 | www.wnus.edu.pl/ab | DOI: 10.18276/ab.2016.23-02 | strony 15–34 The Maria Curie-Skłodowska University Botanical Garden in Lublin as a refuge of the moths (Lepidoptera: Heterocera) within the city Łukasz Dawidowicz,1 Halina Kucharczyk2 Department of Zoology, Maria Curie-Skłodowska University, Akademicka 19, 20-033 Lublin, Poland 1 e-mail: [email protected] 2 e-mail: [email protected] Keywords biodiversity, urban fauna, faunistics, city, species composition, rare species, conservation Abstract In 2012 and 2013, 418 species of moths at total were recorded in the Botanical Garden of the Maria Curie-Skłodowska University in Lublin. The list comprises 116 species of Noctuidae (26.4% of the Polish fauna), 116 species of Geometridae (28.4% of the Polish fauna) and 63 species of other Macrolepidoptera representatives (27.9% of the Polish fauna). The remaining 123 species were represented by Microlepidoptera. Nearly 10% of the species were associated with wetland habitats, what constitutes a surprisingly large proportion in such an urbanised area. Comparing the obtained data with previous studies concerning Polish urban fauna of Lepidoptera, the moths assemblages in the Botanical Garden were the most similar to the one from the Natolin Forest Reserve which protects the legacy of Mazovian forests. Several recorded moths appertain to locally and rarely encountered species, as Stegania cararia, Melanthia procellata, Pasiphila chloerata, Eupithecia haworthiata, Horisme corticata, Xylomoia graminea, Polychrysia moneta. In the light of the conducted studies, the Botanical Garden in Lublin stands out as quite high biodiversity and can be regarded as a refuge for moths within the urban limits of Lublin. -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

INSECTA MUNDIA Journal of World Insect Systematics
INSECTA MUNDI A Journal of World Insect Systematics 0506 Annotated checklist and biogeographic composition of the Lycaenidae (Lepidoptera) of Trinidad, West Indies Matthew J.W. Cock CABI, Bakeham Lane Egham, Surrey, TW20 9TY United Kingdom Robert K. Robbins Smithsonian Institution PO Box 37012, NHB Stop 105 (E-514) Washington, DC 20013-7012 USA Date of Issue: October 21, 2016 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Matthew J.W. Cock and Robert K. Robbins Annotated checklist and biogeographic composition of the Lycaenidae (Lepidoptera) of Trinidad, West Indies Insecta Mundi 0506: 1–33 ZooBank Registered: urn:lsid:zoobank.org:pub:37A7668A-0D83-4DB0-BD28-C36302F18398 Published in 2016 by Center for Systematic Entomology, Inc. P. O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non-marine arthropod. Topics considered for publication include systematics, taxonomy, nomenclature, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medical entomology, pest control research, etc.), and no longer publishes book reviews or editorials. Insecta Mundi publishes original research or discoveries in an inexpensive and timely manner, distributing them free via open access on the internet on the date of publication. Insecta Mundi is referenced or abstracted by several sources including the Zoological Record, CAB Ab- stracts, etc. Insecta Mundi is published irregularly throughout the year, with completed manuscripts assigned an individual number. Manuscripts must be peer reviewed prior to submission, after which they are reviewed by the editorial board to ensure quality. -

Butterflies and Moths of San Bernardino County, California
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

The Taxonomic Report of the INTERNATIONAL LEPIDOPTERA SURVEY
Volume 7 1 February 2010 Number 3 The Taxonomic Report OF THE INTERNATIONAL LEPIDOPTERA SURVEY TIPS ON COLLECTING AND REARING IMMATURES OF 375 BUTTERFLY AND SKIPPER TAXA JACQUE WOLFE 459 East 2700 South Apt 16, Salt Lake City, UT 84115 JACK HARRY 47 San Rafael Court, West Jordan, UT 84088 TODD STOUT 1 1456 North General Drive, Salt Lake City, UT 84116 ABSTRACT: Rearing techniques are discussed for 375 different butterfly and skipper taxa from Utah and beyond. Additional keywords: ova, larvae, pupae, over wintering, obtaining and caring for immatures INTRODUCTION The authors of this paper, Jacque Wolfe, Jack Harry, and Todd Stout, with contributions from Dale Nielson have over 100 years combined experience collecting and rearing butterflies. This publication includes natural and lab host plants. We hope that this information will help you avoid some of the mistakes and losses we have experienced. We also hope that this publication will encourage someone who has only collected adults to give rearing a try. For those new to rearing we encourage starting small. Not only can rearing provide perfect specimens but also provide knowledge regarding the life histories of butterflies, which includes how to find caterpillars or how to entice live females to lay eggs. The advantages justify the time and effort it requires. Another advantage of rearing is that some species, like Papilio indra and Megathymus species, are difficult to collect as adults. Therefor, rearing them can be much easier. For example, collecting larvae or netting a single live female can result in obtaining a nice series of perfect specimens. -

Contribution to the Knowledge of the Fauna of Bombyces, Sphinges And
driemaandelijks tijdschrift van de VLAAMSE VERENIGING VOOR ENTOMOLOGIE Afgiftekantoor 2170 Merksem 1 ISSN 0771-5277 Periode: oktober – november – december 2002 Erkenningsnr. P209674 Redactie: Dr. J–P. Borie (Compiègne, France), Dr. L. De Bruyn (Antwerpen), T. C. Garrevoet (Antwerpen), B. Goater (Chandlers Ford, England), Dr. K. Maes (Gent), Dr. K. Martens (Brussel), H. van Oorschot (Amsterdam), D. van der Poorten (Antwerpen), W. O. De Prins (Antwerpen). Redactie-adres: W. O. De Prins, Nieuwe Donk 50, B-2100 Antwerpen (Belgium). e-mail: [email protected]. Jaargang 30, nummer 4 1 december 2002 Contribution to the knowledge of the fauna of Bombyces, Sphinges and Noctuidae of the Southern Ural Mountains, with description of a new Dichagyris (Lepidoptera: Lasiocampidae, Endromidae, Saturniidae, Sphingidae, Notodontidae, Noctuidae, Pantheidae, Lymantriidae, Nolidae, Arctiidae) Kari Nupponen & Michael Fibiger [In co-operation with Vladimir Olschwang, Timo Nupponen, Jari Junnilainen, Matti Ahola and Jari- Pekka Kaitila] Abstract. The list, comprising 624 species in the families Lasiocampidae, Endromidae, Saturniidae, Sphingidae, Notodontidae, Noctuidae, Pantheidae, Lymantriidae, Nolidae and Arctiidae from the Southern Ural Mountains is presented. The material was collected during 1996–2001 in 10 different expeditions. Dichagyris lux Fibiger & K. Nupponen sp. n. is described. 17 species are reported for the first time from Europe: Clostera albosigma (Fitch, 1855), Xylomoia retinax Mikkola, 1998, Ecbolemia misella (Püngeler, 1907), Pseudohadena stenoptera Boursin, 1970, Hadula nupponenorum Hacker & Fibiger, 2002, Saragossa uralica Hacker & Fibiger, 2002, Conisania arida (Lederer, 1855), Polia malchani (Draudt, 1934), Polia vespertilio (Draudt, 1934), Polia altaica (Lederer, 1853), Mythimna opaca (Staudinger, 1899), Chersotis stridula (Hampson, 1903), Xestia wockei (Möschler, 1862), Euxoa dsheiron Brandt, 1938, Agrotis murinoides Poole, 1989, Agrotis sp. -

Lepidoptera Recorded for Imperial County California Compiled by Jeffrey Caldwell [email protected] 1-925-949-8696 Note
Lepidoptera Recorded for Imperial County California Compiled by Jeffrey Caldwell [email protected] 1-925-949-8696 Note: BMNA = Butterflies and Moths of North America web site MPG = Moth Photographers Group web site Most are from the Essig Museum’s California Moth Specimens Database web site Arctiidae. Tiger and Lichen Moths. Apantesis proxima (Notarctia proxima). Mexican Tiger Moth. 8181 [BMNA] Ectypia clio (clio). Clio Tiger Moth. 8249 Estigmene acrea (acrea). Salt Marsh Moth. 8131 Euchaetes zella. 8232 Autostichidae (Deoclonidae). Oegoconia novimundi. Four-spotted Yellowneck Moth. 1134 (Oegoconia quadripuncta mis-applied) Bucculatricidae. Ribbed Cocoon-maker Moths. Bucculatrix enceliae. Brittlebrush Moth. 0546 Cossidae. Goat Moths, Carpenterworm Moths, and Leopard Moths. Comadia henrici. 2679 Givira mucida. 2660 Hypopta palmata. 2656 Prionoxystus robiniae (mixtus). Carpenterworm or Locust Borer. 2693 Depressariidae. Pseudethmia protuberans. 1008 [MPG] Ethmiidae. Now assigned to Depressariidae. Ethmiinae. Ethmia timberlakei. 0984 Pseudethmia protuberans. 1008 Gelechiidae. Twirler Moths. Aristotelia adceanotha. 1726 [Sighting 1019513 BMNA] Chionodes abdominella. 2054 Chionodes dentella. 2071 Chionodes fructuaria. 2078 Chionodes kincaidella. 2086 (reared from Atriplex acanthocarpa in Texas) Chionodes oecus. 2086.2 Chionodes sistrella. 2116 Chionodes xanthophilella. 2125 Faculta inaequalis. Palo Verde Webworm. 2206 Friseria cockerelli. Mesquite Webworm. 1916 Gelechia desiliens. 1938 Isophrictis sabulella. 1701 Keiferia lycopersicella. Tomato Pinworm. 2047 Pectinophora gossypiella. Pink Bollworm. 2261 Prolita puertella. 1895 Prolita veledae. 1903 Geometridae. Inchworm Moths, Loopers, Geometers, or Measuring Worms. Archirhoe neomexicana. 7295 Chesiadodes coniferaria. 6535 Chlorochlamys appellaria. 7073 Cyclophora nanaria. Dwarf Tawny Wave. W 7140 Dichorda illustraria. 7055 Dichordophora phoenix. Phoenix Emerald. 7057 Digrammia colorata. Creosote Moth. 6381 Digrammia irrorata (rubricata). 6395 Digrammia pictipennata. 6372 Digrammia puertata. -
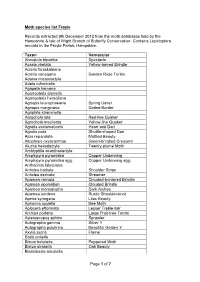
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Proceedings of the Tenth Forum Herbulot 2018. Integrative Taxonomy, a Multidisciplinary Approach to Answer Compli- Cated Taxonomic Questions
SPIXIANA 42 2 291-320 München, Dezember 2019 ISSN 0341-8391 Proceedings of the tenth FORUM HERBULOT 2018. Integrative taxonomy, a multidisciplinary approach to answer compli- cated taxonomic questions (Stuttgart, Germany, 11-16 June 2018) Axel Hausmann & Hossein Rajaei (eds) Hausmann, A. & Rajaei, H. (eds) 2019. Proceedings of the tenth FORUM HERBULOT 2018. Integrative taxonomy, a multidisciplinary approach to answer complicated taxonomic questions (Stuttgart, Germany, 11-16 June 2018). Spixiana 42 (2): 291- 320. The tenth International Congress of FORUM HERBULOT on “Integrative taxonomy, a multidisciplinary approach to answer complicated taxonomic questions” took place in the Staatliches Museum für Naturkunde Stuttgart (SMNS), from 11.- 16.06.2018, with 77 participants and 52 scientific presentations. The proceedings provide short information on the meeting and the abstracts of the oral presenta- tions. Axel Hausmann (corresponding author), SNSB – ZSM, Bavarian State Collection of Zoology, Münchhausenstr. 21, 81247 Munich, Germany; e-mail: [email protected] Short report and results Axel Hausmann & Hossein Rajaei Hausmann, A. & Rajaei, H. 2019. Short report and results. Pp. 291-292 in: Hausmann, A. & Rajaei, H. (eds). Proceedings of the tenth FORUM HERBULOT 2018. Integrative taxonomy, a multidisciplinary approach to answer complicated taxonomic questions (Stuttgart, Germany, 11-16 June 2018). Spixiana 42 (2). Axel Hausmann (corresponding author), SNSB – ZSM, Bavarian State Collection of Zoology, Münchhausenstr. 21, 81247 Mu- nich, Germany; e-mail: [email protected] The meeting was organized by an organization The conference started with a lecture on the ground- team of the ‘Staatliches Museum für Naturkunde breaking effects of “Willi Hennig and the synthesis of Stuttgart’ (SMNS). -

Check List Lists of Species Check List 11(6): 1813, 15 December 2015 Doi: ISSN 1809-127X © 2015 Check List and Authors
11 6 1813 the journal of biodiversity data 15 December 2015 Check List LISTS OF SPECIES Check List 11(6): 1813, 15 December 2015 doi: http://dx.doi.org/10.15560/11.6.1813 ISSN 1809-127X © 2015 Check List and Authors Butterflies (Lepidoptera: Papilionoidea and Hesperioidea) of the Banhado dos Pachecos Wildlife Refuge, Uruguayan Savanna Ecoregion, Rio Grande do Sul state, Brazil Andressa Caporale1, Liana Bertoldi Moreno1, Nicolás Oliveira Mega1, 2*, Helena Piccoli Romanowski1, 2 1 Graduate Program in Animal Biology. Federal University of Rio Grande do Sul. Av. Bento Gonçalves 9500/43435. CEP 91504-970. Porto Alegre, RS, Brazil 2 Department of Zoology. Federal University of Rio Grande do Sul. Av. Bento Gonçalves 9500/43435. CEP 91504-970. Porto Alegre, RS, Brazil * Corresponding author. E-mail: [email protected] Abstract: The Pampa is a biome shared by Argentina, the Pampa presents high biodiversity and is home to Brazil, and Uruguay. Despite its high biodiversity, little is very characteristic flora and fauna. Estimates indicate known about the invertebrate fauna. The few inventories the presence of approximately 3,000 plant species, over done so far were conducted outside protected areas, 100 mammal species, and almost 500 bird species (MMA which could result in underestimated real biodiversity. 2007). Thus, species inventories from protected areas should Despite its high biodiversity, little is known about be done to serve as reference for conservation. Here invertebrate diversity in the Pampa. Considering that we survey the butterflies occurring in the Banhado dos only recent studies focus on non-pest insects, some Pachecos Wildlife Refuge, Uruguayan Savanna, Brazil. -

Act Native Woodland Conservation Strategy and Action Plans
ACT NATIVE WOODLAND CONSERVATION STRATEGY AND ACTION PLANS PART A 1 Produced by the Environment, Planning and Sustainable Development © Australian Capital Territory, Canberra 2019 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without written permission from: Director-General, Environment, Planning and Sustainable Development Directorate, ACT Government, GPO Box 158, Canberra ACT 2601. Telephone: 02 6207 1923 Website: www.planning.act.gov.au Acknowledgment to Country We wish to acknowledge the traditional custodians of the land we are meeting on, the Ngunnawal people. We wish to acknowledge and respect their continuing culture and the contribution they make to the life of this city and this region. Accessibility The ACT Government is committed to making its information, services, events and venues as accessible as possible. If you have difficulty reading a standard printed document and would like to receive this publication in an alternative format, such as large print, please phone Access Canberra on 13 22 81 or email the Environment, Planning and Sustainable Development Directorate at [email protected] If English is not your first language and you require a translating and interpreting service, please phone 13 14 50. If you are deaf, or have a speech or hearing impairment, and need the teletypewriter service, please phone 13 36 77 and ask for Access Canberra on 13 22 81. For speak and listen users, please phone 1300 555 727 and ask for Canberra Connect on 13 22 81. For more information on these services visit http://www.relayservice.com.au PRINTED ON RECYCLED PAPER CONTENTS VISION ........................................................................................................... -

ACT, Australian Capital Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations.