Article Download (110)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Belgaum District Lists
Group "C" Societies having less than Rs.10 crores of working capital / turnover, Belgaum District lists. Sl No Society Name Mobile Number Email ID District Taluk Society Address 1 Abbihal Vyavasaya Seva - - Belgaum ATHANI - Sahakari Sangh Ltd., Abbihal 2 Abhinandan Mainariti Vividha - - Belgaum ATHANI - Uddeshagala S.S.Ltd., Kagawad 3 Abhinav Urban Co-Op Credit - - Belgaum ATHANI - Society Radderahatti 4 Acharya Kuntu Sagara Vividha - - Belgaum ATHANI - Uddeshagala S.S.Ltd., Ainapur 5 Adarsha Co-Op Credit Society - - Belgaum ATHANI - Ltd., Athani 6 Addahalli Vyavasaya Seva - - Belgaum ATHANI - Sahakari Sangh Ltd., Addahalli 7 Adishakti Co-Op Credit Society - - Belgaum ATHANI - Ltd., Athani 8 Adishati Renukadevi Vividha - - Belgaum ATHANI - Uddeshagala S.S.Ltd., Athani 9 Aigali Vividha Uddeshagala - - Belgaum ATHANI - S.S.Ltd., Aigali 10 Ainapur B.C. Tenenat Farming - - Belgaum ATHANI - Co-Op Society Ltd., Athani 11 Ainapur Cattele Breeding Co- - - Belgaum ATHANI - Op Society Ltd., Ainapur 12 Ainapur Co-Op Credit Society - - Belgaum ATHANI - Ltd., Ainapur 13 Ainapur Halu Utpadakari - - Belgaum ATHANI - S.S.Ltd., Ainapur 14 Ainapur K.R.E.S. Navakarar - - Belgaum ATHANI - Pattin Sahakar Sangh Ainapur 15 Ainapur Vividha Uddeshagal - - Belgaum ATHANI - Sahakar Sangha Ltd., Ainapur 16 Ajayachetan Vividha - - Belgaum ATHANI - Uddeshagala S.S.Ltd., Athani 17 Akkamahadevi Vividha - - Belgaum ATHANI - Uddeshagala S.S.Ltd., Halalli 18 Akkamahadevi WOMEN Co-Op - - Belgaum ATHANI - Credit Society Ltd., Athani 19 Akkamamhadevi Mahila Pattin - - Belgaum -

Prl. District and Session Judge, Belagavi. SRI. BASAVARAJ I ADDL
Prl. District and Session Judge, Belagavi. SRI. BASAVARAJ I ADDL. DISTRICT AND SESSIONS JUDGE BELAGAVI Cause List Date: 18-09-2020 Sr. No. Case Number Timing/Next Date Party Name Advocate 1 M.A. 8/2020 Moulasab Maktumsab Sangolli A.D. (HEARING) Age 70Yrs R/o Bailhongal Dist SHILLEDAR IA/1/2020 Belagavi. Vs The Chief officer Bailhongal Town Municipal Council Tq Bailhongal Dist Belagavi. 2 L.A.C. 607/2018 Laxman Dundappa Umarani age C B Padnad (EVIDENCE) 65 Yrs R/o Kesaral Tq Athani Dt Belagavi Vs The SLAO Hipparagi Project , Athani Dist Belagavi. 3 L.A.C. 608/2018 Babalal Muktumasab Biradar C B Padanad (EVIDENCE) Patil Age 55 yrs R/o Athani Tq Athani Dt Belagavi. Vs The SLAO Hipparagi Project , Athani, Tq Athani Dist Belagavi. 4 L.A.C. 609/2018 Gadigeppa Siddappa Chili age C B padanad (EVIDENCE) 65 Yrs R/o Athani Tq Athani Dt Belagavi Vs The SLAO Hipparagi Project , Athani Dist Belagavi. 5 L.A.C. 610/2018 Kedari Ningappa Gadyal age 45 C B Padanad (EVIDENCE) Yrs R/o Athani Tq Athani Dt Belagavi Vs The SLAO Hipparagi Project , Athani Dist Belagavi. 6 L.A.C. 611/2018 Smt Kallawwa alias Kedu Bhima C B padanad (EVIDENCE) Pujari Vs The SLAO Hipparagi Project , Athani Dist Belagavi. 7 L.A.C. 612/2018 Kadappa Bhimappa Shirahatti C B Padanad (EVIDENCE) age 55 Yrs R/o Athani Tq Athani Dt Belagavi Vs The SLAO Hipparagi Project , Athani. Dist Belagavi. 1/8 Prl. District and Session Judge, Belagavi. SRI. BASAVARAJ I ADDL. DISTRICT AND SESSIONS JUDGE BELAGAVI Cause List Date: 18-09-2020 Sr. -

Prl. District and Session Judge, Belagavi. Sri
Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL. DISTRICT AND SESSIONS JUDGE BELAGAVI Cause List Date: 18-12-2020 Sr. No. Case Number Timing/Next Date Party Name Advocate 11.00 AM-02.00 PM 1 SC 20/2020 The State of Karnataka R/by PP, PP (APPEARANCE OF Belagavi. ACCUSED) Vs Rajan Manohar Kakade Age. 56 years R/o H.No.1978, Siddannavar Complex Ganapati Galli, Belagavi. 2 Crl.Misc. 1857/2020 Pandappa Venkappa Chavalar, R.C.PATIL (OBJECTION) Age 72yrs, R/o Ramdurg Tq Ramadurg Dist Belagavi Vs The State of Karnataka, R/by its P.P.Belagavi 2.45 PM- 5.45 PM 3 Crl.Misc. 1841/2020 Shanta Shivanand Patil @ B S DHADED (ORDERS) Shanta Chandrakant Kyadigumpi, Age 39,R/o.APMC, Ilakal,Tq.Hunagund, Vs The State of Karnataka, R/by P.P.Belagavi 4 Crl.Misc. 1838/2020 Ramappa Tayi Karevva Metri @ Kannoli (ORDERS) Madar, Age 50yrs, R/o.Hoskoti, Prashant S. Tq.Ramdurg, Dist.Belagavi Vs The State of Karnataka, R/by P.P.Belagavi 1/1 Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL. DISTRICT AND SESSIONS JUDGE BELAGAVI Cause List Date: 18-12-2020 Sr. No. Case Number Timing/Next Date Party Name Advocate 11.00 AM-02.00 PM 1 R.A. 349/2019 Anusuya Wd/o Yallappa Songadi Desai Mahesh S (NOTICE) Age 80 yrs R/o Kamat Galli,Tq IA/1/2019 and Dt Belagavi. Vs Gyaneshwar Shatuppa Mutagekar Age 56 yrs R/o H.No.27,Vithaldev Galli,Kallehol,Tq and Dt Belagavi. -

29/01/2020 Government of Karnataka Page:103
29/01/2020 GOVERNMENT OF KARNATAKA PAGE:103 DEPARTMENT OF PRE UNIVERSITY EDUCATION LIST OF PU COLLEGES IN BELGAUM DISTRICT AS ON 29/01/2020 ******************************************************************************** SLNO COLCD NAME AND ADDRESS YEAR OF OPEN & COLL TYPE OPENING & AIDED GO NOS. WITH DATE ******************************************************************************** 1377 DD001 LINGARAJ A&C PU COLLEGE 33-34 BIFUR PU COL COLLEGE ROAD BELGAUM 590001 -------------------------------------------------------------------------------- 1378 DD002 GILGANCHI ARTAL PU COL 72-73 AIDED PU COL BELGAUM ED 19 TPU 72 DT 16-05-1972 BELGAUM 590001 PLG1/593-PUC-6/72-73 DT 02/08/1972 -------------------------------------------------------------------------------- 1379 DD003 GOVT SARDAR'S PU COLLEGE 72-73 GOVT PU COL KAKATIVES ROAD ED 102 TPU 72 DT 06-02-1973 BELGAUM 590002 -------------------------------------------------------------------------------- 1380 DD004 RAJA LAKHAMAGOUDA PU COL 58-59 BIFUR PU COL COLLEGE ROAD BELGAUM 590001 -------------------------------------------------------------------------------- 1381 DD005 BEYNON SMITH COMP PU COL 72-73 AIDED PU COL CAMP PLG1-593-PUC6 72-73A BELGAUM 590001 PLG1/JCM-5/77-78 DT 13/10/1977 -------------------------------------------------------------------------------- 1382 DD006 RANI PARVATHIDEVI PU COL BIFUR PU COL TILAKWADI BELGAUM 590006 -------------------------------------------------------------------------------- 1383 DD007 GOVINDRAM SEKSARIA PU COL BIFUR PU COL TILAKWADI RPD COLLEGE -

Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL
Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL. DISTRICT AND SESSIONS JUDGE BELAGAVI Cause List Date: 24-11-2020 Sr. No. Case Number Timing/Next Date Party Name Advocate 11.00 AM-02.00 PM 1 R.A. 576/2019 Demappa Gangappa Bandagi H.C.Savasuddi (HEARING) Age 75 yrs R/o IA/1/2019 Inchal,Tq.Saundatti,Dt.Belagavi. IA/2/2019 Vs IA/3/2019 Shivappa Gangappa Bandagi Age 67 yrs R/o Inchal,Tq.Saundatti,Dt.Belagavi. 2 M.A. 24/2020 The Belgaum Janata Shikshana A.M.Potdar (HEARING) Samiti Now Bharatesh Education Trust R/by A.S.Somannavar V.S.Doddannavar R/o Belagavi Vs The Cantonment Board,BGV R/by the Honble Chief Executive officer office/situated at Khanapur Rd,BGV 3 M.A. 25/2020 The Belgaum Janata Shikshana A.M.Potdar (HEARING) Samiti Now Bhartesh Education Trust R/by V.S.Doddannavar R/o A.S.Somannavar Belagavi. Vs The Cantonment Board,BGV R/by the Honble Chief Execution officer office situated at Khanpur Rd,BGV 4 M.A. 26/2020 Dakshin MH. Shikshan Mandals P. S. Patil (HEARING) Jyoti Central School, Camp BGV R/by V.L.Patil R/o Shivaji A.S.Somannavar road,Belagvi Vs The Cantonment Board Belagaum R/by Chief Executive Officer Khanapur Road, Camp Belagavi. 5 M.A. 27/2020 Dakshin MH.Shikshan Mandals PP.S Patil (HEARING) Marathi Vidya Niketan, Jyoti Compounded R/by V.L.Patil R/o A.S.Somannavar Belagavi. Vs The Cantonment Board, Belagavi R/by Chief Executive Officer Khanapur Road Camp, Belagavi 1/3 Prl. -

KUVEMPU UNIVERSITY DIRECTORATE of DISTANCE EDUCATION Details of the Admissions Made for the Academic Session 2018-19 (January, 2019) Under Open and Distance Learning
KUVEMPU UNIVERSITY DIRECTORATE OF DISTANCE EDUCATION Details of the admissions made for the academic session 2018-19 (January, 2019) under Open and Distance Learning 1. Programme-wise details a) Bachelor of ARTS Government Issued Category Date of Identifier ( eg: AADHAR Sl.No. Name of the students Enrolment No. SC/ST/OBC/PWD*/E Contact Details (Ph. No. , email id etc) admission Card / PAN Card / Voter Id WS** Card No. etc.) 1 2 3 4 5 6 7 8618257212 [email protected] #481, 8th cross Anada Puram Jeevan Bima Nagar, New 1 A ANNU MARLIN DUBA155050033 05-02-2019 OBC 872265455218 thippasandra post Bangalore 560075 Bangalore Karnataka 8970232598 [email protected] HOSA EXTENSTION TARIKERE TALUK 2 ABHILASH K K DUBA155010198 07-02-2019 OBC 414839226944 MALALICHANNENAHALLI 577228 CHICKMAGALURE KARNATAKA 9606609532 [email protected] C/O CHANDRASHEKAR 4TH A BLOCK P V 3 AKASHA K DUBA155011508 28-02-2019 SC KRISHNAMURTHY BADAVANE JYOTHIRAO 341107097776 STREET VIDYANAGARA SHIMOGA 577203 8296969932SHIMOGA KARNATAKA [email protected] 4 AKKAMAHADEVI YATNATTI DUBA155010125 03-02-2019 OBC AKKAMAHADEVI YATNATTI A/P- YARANAL 583363841235 TQ- B BAGEWADI 586122 VIJAYAPUR KARNATAKA 9902564464 [email protected] AKSHATA C 5 AKSHATA C DULARI DUBA155011315 26-02-2019 SC DULARI APMC QUARTERS NO-05 INDI ROAD 245678323863 586101 VIJAYAPUR KARNATAKA 8762483905 [email protected] 91 KUNDURU YELEMADALU VILLAGE 6 AKSHATHA J DUBA155050072 12-02-2019 OBC 472237842704 DURGADABETTA POST 577118 CHIKKAMAGALURU KARNATAKA 9901032374 [email protected] -

Table of Content of Teachers' Constituency of Legislative Council
Table of Content of Teachers' Constituency of Legislative Council Electoral Roll of the Year 2015 Name Of State: Karnataka Name of Teachers' Constituency : Karnataka North West Teachers DETAILS OF REVISION Year of revision : 2015 Type of revision : Summary Qualifying date : 01-11-2015 Date of publication : 18-01-2016 (a).Name of Teachers' Constituency : Karnataka North West Teachers (b).Districts in which the aforesaid Constituency is located : Belgaum,Bagalkot,Bijapur. (c) No. and Name of Assembly Constituency Comprised within the All the Assembly Constituencies in the districts of aforesaid Teachers' Constituency : Belgaum,Bagalkot,Bijapur. TOTAL NO. OF PARTS IN THE CONSTITUENCY : 150. COMPONENTS OF THE ROLL. (A) Mother roll (B) Supplement-1 (C) Supplement-2 NET NUMBER OF ELECTORS : Male Female Others Total 22107 4850 0 26957 Part No : 53 Electoral Rolls,2015 of North West Teachers Constituency of Karnataka Legislative Council District : Belgaum Taluk/Town/City : Soudatti Area : Yaragatti,Soppadl,Korakoppa,Madlur,Shrirangapur,Mabanur,Kadabi,Yarazaravi, Tallur,Jalika tti,Aladakatti,K.M.Rainapur,Budigoppa,Nugganatti,Sattigeri,Gudamakeri,Kodlivad, ,Akkisagar, Govanakoppa,K.S.Kotur,Madamageri,Yaraganavi,Tavalageri Sl No Name of the Elector Name of Address (Place of Ordinary School/College where Teaching Age Sex EPIC Photo of Father/Mother/Husband Residence) Number the elector 1 2 3 4 5 6 7 8 9 1 Basavaraj Aladkatti Shivappa yaragatti, Yaragatti, 591129 Bapuji Shikshan 49 M BZM1603554 Sansthe,Masarguppi 2 Manjunath Alagodi Shivappa Rainapur, -

Bedkar Veedhi S.O Bengaluru KARNATAKA
pincode officename districtname statename 560001 Dr. Ambedkar Veedhi S.O Bengaluru KARNATAKA 560001 HighCourt S.O Bengaluru KARNATAKA 560001 Legislators Home S.O Bengaluru KARNATAKA 560001 Mahatma Gandhi Road S.O Bengaluru KARNATAKA 560001 Rajbhavan S.O (Bangalore) Bengaluru KARNATAKA 560001 Vidhana Soudha S.O Bengaluru KARNATAKA 560001 CMM Court Complex S.O Bengaluru KARNATAKA 560001 Vasanthanagar S.O Bengaluru KARNATAKA 560001 Bangalore G.P.O. Bengaluru KARNATAKA 560002 Bangalore Corporation Building S.O Bengaluru KARNATAKA 560002 Bangalore City S.O Bengaluru KARNATAKA 560003 Malleswaram S.O Bengaluru KARNATAKA 560003 Palace Guttahalli S.O Bengaluru KARNATAKA 560003 Swimming Pool Extn S.O Bengaluru KARNATAKA 560003 Vyalikaval Extn S.O Bengaluru KARNATAKA 560004 Gavipuram Extension S.O Bengaluru KARNATAKA 560004 Mavalli S.O Bengaluru KARNATAKA 560004 Pampamahakavi Road S.O Bengaluru KARNATAKA 560004 Basavanagudi H.O Bengaluru KARNATAKA 560004 Thyagarajnagar S.O Bengaluru KARNATAKA 560005 Fraser Town S.O Bengaluru KARNATAKA 560006 Training Command IAF S.O Bengaluru KARNATAKA 560006 J.C.Nagar S.O Bengaluru KARNATAKA 560007 Air Force Hospital S.O Bengaluru KARNATAKA 560007 Agram S.O Bengaluru KARNATAKA 560008 Hulsur Bazaar S.O Bengaluru KARNATAKA 560008 H.A.L II Stage H.O Bengaluru KARNATAKA 560009 Bangalore Dist Offices Bldg S.O Bengaluru KARNATAKA 560009 K. G. Road S.O Bengaluru KARNATAKA 560010 Industrial Estate S.O (Bangalore) Bengaluru KARNATAKA 560010 Rajajinagar IVth Block S.O Bengaluru KARNATAKA 560010 Rajajinagar H.O Bengaluru KARNATAKA -

Name and Address of the Booth Level Officers Name of the Assembly Constituency : 01-NIPANI Total No
Name and Address of the Booth Level Officers Name of the Assembly Constituency : 01-NIPANI Total No. of parts in Assembly Constituency : 167 Total No. of B.L.Os in the Assembly Constituency : 167 Taluka : CHIKODI District : BELGAUM Desig Part Name of the BLO nation Complete address of the BLO Contact No. No. (T/NT) 1Q 2 3 4 5 1 Shri. Venkatesh Sagar T A/P: Sulagaon Tq: Chikodi 9740638089 2 Shri. P.H. Patil NT A/P: Koganolli Tq: Chikodi 9945392089 3 Shri. M. R Hudedamani T A/P: Koganolli Tq: Chikodi 9449535560 9535654218 4 Shri. M. H. Walake T A/P: Koganolli Tq: Chikodi 9421204584 5 Shri.D.D.Dhobale T A/P: Koganolli Tq: Chikodi 9886725251 942322117 6 Shri. H.M. Rawutappa T A/P:Koganolli Tq: Chikodi 9449171737 7 Shri. B.D. Dohale T A/P:Koganolli Tq: Chikodi 9423282117 8 Shri. A.N. Nadaf T A/P:Koganolli Tq: Chikodi 9449938401 9 Shri. S. M. Shinde T A/P: Hanabarwadi Tq: Chikodi 9326412753 10 Shri. R. S. Chennadasar T A/P: Hanchinal K.S Tq: Chikodi 9886444529 9986053276 11 Shri. S. B. Sarwad T A/P: Hanchinal K.S Tq: Chikodi 9986478524 12 Shri. L. B. Pujari NT A/P: Kunnur. Tq: Chikodi 9742118565 9742364429 13 Shri. D.T. Kamble T A/P: Kunnur Tq: Chikodi 9008928638 14 Shri. N.B. Bhimanagouda T A/P: Kunnur Tq: Chikodi 9880564480 15 Shri. M.K. Talawar NT A/P: Kunnur Tq: Chikodi 9902789877 16 Shri. S.K. Badaganve, T A/P: Kunnur Tq: Chikodi 08338 - 294007 9620090407 17 Shri. -

Cxภtย Vมฎฦqฃภ 51 Uมๆชภฤ ฅภazมaiภฤwuภฝuษ ฑมธภฃภงzภ Cฃภฤzมฃภ Dฃ๏ ฏษสฃ๏ ชภฤฦฎPภ ©Qภฤuภqษ ชภiมqภฤชภ §Uษฮ จมๅAQฃภ ซชภgภ
CxÀt vÁ®ÆQ£À 51 UÁæªÀÄ ¥ÀAZÁAiÀÄwUÀ½UÉ ±Á¸À£À§zÀÝ C£ÀÄzÁ£À D£ï ¯ÉÊ£ï ªÀÄÆ®PÀ ©qÀÄUÀqÉ ªÀiÁqÀĪÀ §UÉÎ ¨ÁåAQ£À «ªÀgÀ. Taluka Panchayat Athani Name of the Name of the Name of the Name of Branch IFSC Name as PDO Mobile Sl No Gram Account Number Amount GP`s E-mail Adress District Taluka the Bank Code Number Panchayat Name per Bank 12 3456 7 8 9 10 11 12 1 Belgaum Athani Adahalli K.V.G.B Adahalli 1721847007-8 2001 80000 9972430720 [email protected] 2 Aigali K.V.G.B Adahalli 1721846673-2 2001 100000 9686267227 [email protected] 3 Aralihatti K.V.G.B Madbhavi 1721705653-4 2007 80000 9972707837 [email protected] 4 Artal K.V.G.B Telsang 1718954664-1 2013 80000 8095013223 [email protected] 5 Balligeri K.V.G.B Athani 1718806392-6 2002 100000 9663570739 [email protected] 6 Darur K.V.G.B Halyal 8901713753-8 2003 80000 9480854208 [email protected] 7 Gundewadi K.V.G.B Athani 1718806394-8 2002 80000 9164079709 [email protected] 8 Halyal K.V.G.B Halyal 1718854571-8 2003 80000 9900345922 [email protected] 9 Hulagabali K.V.G.B Athani 1718806404-5 2002 80000 8105399169 [email protected] 10 Jambagi K.V.G.B Athani 1718806396-0 2002 100000 9481654612 [email protected] 11 Junjarwad K.V.G.B Kokatanur 1718903282-8 2006 80000 8095737880 [email protected] 12 Kagawad K.V.G.B Kagawad 1718352453-3 2004 100000 9880012306 [email protected] 13 Kakamari K.V.G.B Telsang 1718955287-1 2013 100000 9663570739 [email protected] 14 Kempwad K.V.G.B Mangasuli 1717555035-8 2008 80000 9731101707 [email protected] 15 Khilegaon K.V.G.B Khilegaon 1718702125-1 -

Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL
Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL. DISTRICT AND SESSIONS JUDGE BELAGAVI Cause List Date: 06-10-2020 Sr. No. Case Number Timing/Next Date Party Name Advocate 2.45 PM- 5.45 PM 1 Crl.Misc. 1528/2020 Rayappa S/o Bhimappa Khot Age P.K.HUKKERIMATH (ORDERS) 60yrs R/o Mirapurhatti Tq Chikkodi Dt Belagavi Vs The State of Karnataka Chikkodi PS Rb/y PP Belagavi 2 Crl.Misc. 1543/2020 Umesh Muttappa Bevanur Age A.K.Ingale and (ORDERS) 25Yrs R/o Mavinhatti, Abbihal P.R.Rodabasannavar. Tq Athani Dist Belagavi. Vs The State of Karnataka R/by Its P.P. Belagavi. 1/1 Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL. DISTRICT AND SESSIONS JUDGE BELAGAVI Cause List Date: 06-10-2020 Sr. No. Case Number Timing/Next Date Party Name Advocate 11.00 AM-02.00 PM 1 R.A. 576/2019 Demappa Gangappa Bandagi H.C.Savasuddi (NOTICE) Age 75 yrs R/o IA/1/2019 Inchal,Tq.Saundatti,Dt.Belagavi. IA/2/2019 Vs IA/3/2019 Shivappa Gangappa Bandagi Age 67 yrs R/o Inchal,Tq.Saundatti,Dt.Belagavi. 2 A.S. 9/2018 Rajappa Laxman Bajantri Age Y. G. GUMAJ, (SUMMONS) 45yrs R/o B No , Room NO 149, IA/1/2018 Dar Police Head Quaters S P KULKARNI Belagavi Vs The Shriram Transport Finance Co R/o 1st Flr CTS No.4801/1/A, Behind RTO Office P.B.Road BGV 3 Misc 101/2020 Competent Authority and Sub A.B.BASAPURE (APPEARANCE OF Divisional Magistrate Belagavi PARTY) Sub Div. -
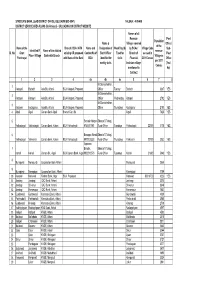
Sl. No. Name of the Gram Panchayat Identified FI Place / Village Name Of
SYNDICATE BANK, LEAD DISTRICT OFFICE, BELGAUM (BIC-0541) TALUKA :- ATHANI DISTRICT SERVICE AREA PLANS (Re-Revised) - UPLOADING ON DISTRICT WEBSITE Names of all Revenue Post Population Name & Villages covered Office / of the Name of the Branch / BCA / ATM Name and Designation of Week Day (S) by BCAs / Village Code Sub- Identified FI Name of the allotted revenue Sl. No. Gram existing OR proposed Contact No.of Bank Officer Fixed for Branch of as used in Post Place / Village Bank with Branch Village as Panchayat with Name of the Bank BCA identified for visits Financial 2011 Census Office per 2001 weekly visits Inclusion villages (Yes / Census mentioned in No) Col.No.2 1 2 3 4 4a 4b 4c 5 6 7 8 Mr.Somashekhar, 1 Katageri Badachi Axis Bk, Athani BCA Katageri, Proposed Officer Tueday Badachi 3387 YES Mr.Somashekhar, 2 Katageri Katageri Axis Bk, Athani BCA Katageri, Proposed Officer Wednesday Katageri 2792 YES Mr.Somashekhar, 3 Katageri Kodaganur Axis Bk, Athani BCA Katageri, Proposed Officer Thursdays Kodaganur 2193 YES 4 Aigali Aigali Canara Bank Aigali Branch Can. Bk. Aigali 7425 YES 5 Santosh Magar Shweta S Totagi, Yalihadagali Yalihadagali Canara Bank, Athani BCA Yalihadagali 9740411581 Rural Officer Tuesdays Yalihadagali 22000 3178 YES 6 Basappa Kiwadi Shweta S Totagi, Yalihadagali Yakkanchi Canara Bank, Athani BCA Yalihadagali 9972025353 Rural Officer Thursdays Yakkanchi 22100 2052 YES Appanna 7 Biradar, Shweta S Totagi, Kohalli Kohalli Canara Bk., Aigali BCA Canara Bank, Aigali 9902013573 Rural Officer Tuesdays Kohalli 21600 5948 YES 8