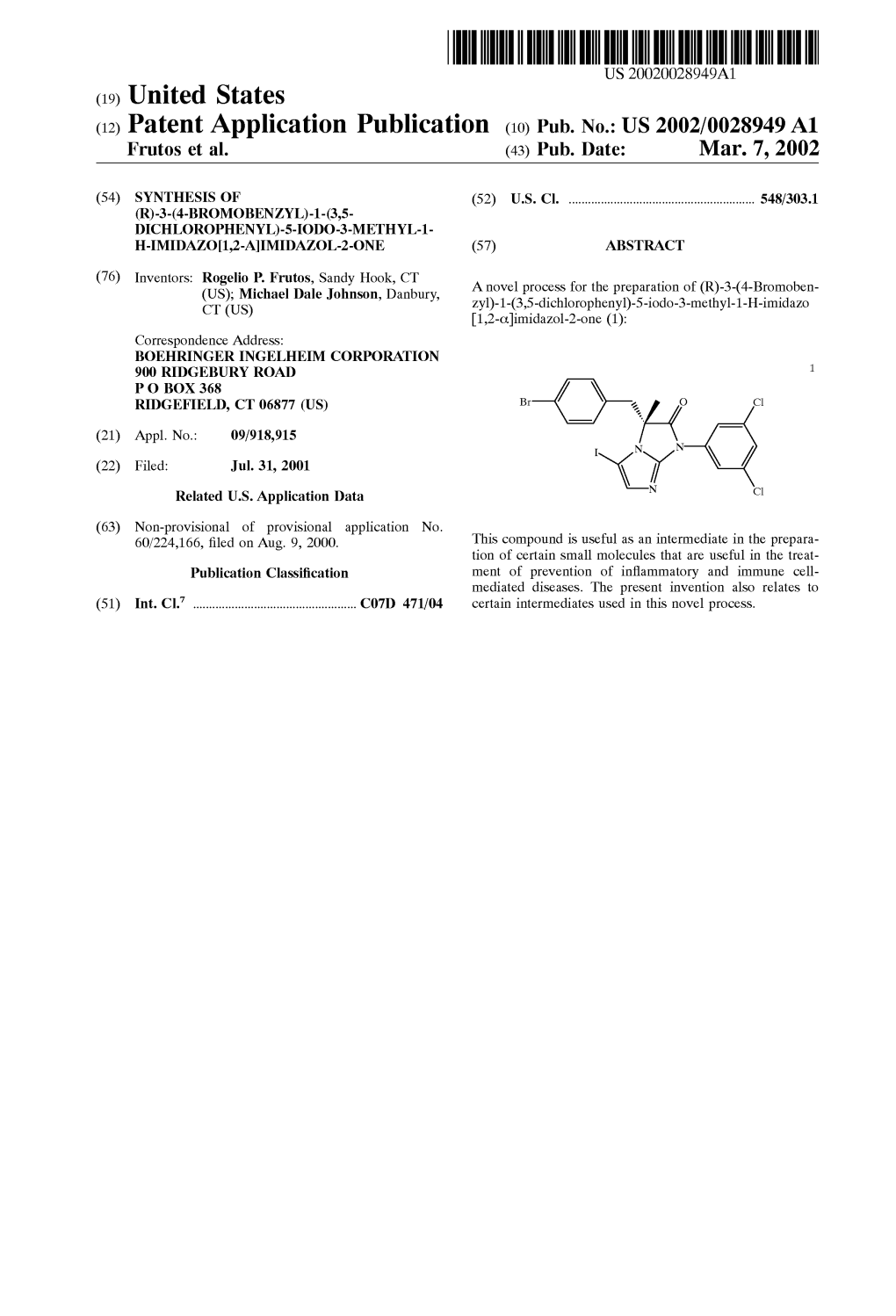
(12) Patent Application Publication (10) Pub. No.: US 2002/0028949 A1 Frutos Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chlorotrimethylsilane
Chlorotrimethylsilane Health-based recommended occupational exposure limit Gezondheidsraad Voorzitter Health Council of the Netherlands Aan de Staatssecretaris van Sociale Zaken en Werkgelegenheid Onderwerp : Aanbieding adviezen over Chloortrimethylsilaan en Butylacetaten Uw kenmerk : DGV/MBO/U-932542 Ons kenmerk : U 2173/AvdB/tvdk/459-D35 Bijlagen : 2 Datum : 15 november 2001 Mijnheer de Staatssecretaris, Bij brief van 3 december 1993, nr. DGV/BMO-U-932542, verzocht de Staatssecretaris van Welzijn, Volksgezondheid en Cultuur namens de Minister van Sociale Zaken en Werkgelegenheid om gezondheidskundige advieswaarden af te leiden ten behoeve van de bescherming van beroepsmatig aan stoffen blootgestelde personen. Per 1 januari 1994 heeft mijn voorganger daartoe een commissie ingesteld die de werkzaamheden voortzet van de Werkgroep van Deskundigen (WGD). De WGD was een door de genoemde Minister ingestelde adviescommissie. Hierbij bied ik u - gehoord de Beraadsgroep Gezondheid en Omgeving - twee publicaties aan van de commissie over chloortrimethylsilaan en butylacetaten. Deze publicaties heb ik heden ter kennisname aan de Minister van Volksgezondheid, Welzijn en Sport en aan de Minister van Volkshuisvesting, Ruimtelijke Ordening en Milieubeheer gestuurd. Hoogachtend, w.g. prof. dr JA Knottnerus Bezoekadres Postadres Parnassusplein 5 Postbus 16052 2511 VX Den Haag 2500 BB Den Haag Telefoon (070) 340 75 20 Telefax (070) 340 75 23 Chlorotrimethylsilane Health-based recommended occupational exposure limit report of the Dutch Expert Committee on Occupational Standards, a committee of the Health Council of The Netherlands to the Minister and State Secretary of Social Affairs and Employment No. 2001/05OSH, The Hague, 15 November 2001 The Health Council of the Netherlands, established in 1902, is an independent scientific advisory body. -

Rubber Composition, Method For
(19) TZZ _T (11) EP 2 674 456 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C08L 15/00 (2006.01) B60C 1/00 (2006.01) 01.11.2017 Bulletin 2017/44 C08C 19/25 (2006.01) C08J 3/20 (2006.01) C08K 3/36 (2006.01) C08K 5/098 (2006.01) (2006.01) (2006.01) (21) Application number: 12744729.0 C08L 9/06 C08C 19/44 C08L 25/10 (2006.01) (22) Date of filing: 09.02.2012 (86) International application number: PCT/JP2012/052928 (87) International publication number: WO 2012/108488 (16.08.2012 Gazette 2012/33) (54) RUBBER COMPOSITION, METHOD FOR PRODUCING SAME, AND TIRE KAUTSCHUKZUSAMMENSETZUNG, VERFAHREN ZU IHRER HERSTELLUNG UND REIFEN COMPOSITION DE CAOUTCHOUC, SON PROCÉDÉ DE PRODUCTION, ET PNEUMATIQUE (84) Designated Contracting States: • OKADA, Koji AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Tokyo 105-8640 (JP) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (74) Representative: TBK Bavariaring 4-6 (30) Priority: 09.02.2011 JP 2011026042 80336 München (DE) (43) Date of publication of application: (56) References cited: 18.12.2013 Bulletin 2013/51 EP-A1- 1 505 087 EP-A1- 1 577 341 EP-A2- 2 009 048 DE-A1-102005 044 998 (73) Proprietor: JSR Corporation JP-A- 2005 008 870 US-A1- 2002 082 333 Tokyo 105-8640 (JP) US-A1- 2010 130 663 US-A1- 2011 003 932 (72) Inventors: • SHIBATA, Masahiro Tokyo 105-8640 (JP) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Silyl Ketone Chemistry. Preparation and Reactions of Silyl Allenol Ethers. Diels-Alder Reactions of Siloxy Vinylallenes Leading to Sesquiterpenes2
J. Am. Chem. SOC.1986, 108, 7791-7800 7791 pyrany1oxy)dodecanoic acid, 1.38 1 g (3.15 mmol) of GPC-CdCIz, 0.854 product mixture was then filtered and concentrated under reduced g (7.0 mmol) of 4-(dimethylamino)pyridine, and 1.648 g (8.0 mmol) of pressure. The residue was dissolved in 5 mL of solvent B and passed dicyclohexylcarbodiimide was suspended in 15 mL of dry dichloro- through a 1.2 X 1.5 cm AG MP-50 cation-exchange column in order to methane and stirred under nitrogen in the dark for 40 h. After removal remove 4-(dimethylamino)pyridine. The filtrate was concentrated under of solvent in vacuo, the residue was dissolved in 50 mL of CH30H/H20 reduced pressure, dissolved in a minimum volume of absolute ethanol, (95/5, v/v) and stirred in the presence of 8.0 g of AG MP-50 (23 OC, and then concentrated again. Chromatographic purification of the res- 2 h) to allow for complete deprotection of the hydroxyl groups (monitored idue on a silica gel column (0.9 X 6 cm), eluting first with solvent A and by thin-layer chromatography)." The resin was then removed by fil- then with solvent C (compound 1 elutes on silica as a single yellow band), tration and the solution concentrated under reduced pressure. The crude afforded, after drying [IO h, 22 OC (0.05 mm)], 0.055 g (90%) of 1 as product (2.75 g). obtained after drying [12 h, 23 OC (0.05 mm)], was a yellow solid: R 0.45 (solvent C); IR (KBr) ucz0 1732, uN(cH3)3 970, then subjected to chromatographic purification by using a 30-g (4 X 4 1050, 1090cm-'; I' H NMR (CDCI,) 6 1.25 (s 28 H, CH2), 1.40-2.05 (m, cm) silica gel column, eluting with solvents A and C, to yield 0.990 g 20 H, lipoic-CH,, CH2CH20,CH2CH,C02), 2.3 (t. -

A Novel Procedure for Qualifying and Quantifying Mono-, Di-, And
A Novel Procedure for Qualifying and Quantifying Mono-, Di-, and Trisaccharides by Derivatization and Gas Chromatography A Senior Honors Thesis Presented in Partial Fulfillment of the Requirements for Graduation With Research Distinction in the Undergraduate Colleges of The Ohio State University By Xiaxi Liu The Ohio State University June 2011 Project Advisor: Dr. Christopher Callam, Department of Chemistry Thesis Committee: Dr. David Stetson, Department of Evolution, Ecology, and Organismal Biology. Dr. Gary Means, Department of Biochemistry Dr. Madhura Pradhan, Department of Microbiology Table of Contents Acknowledgements ...................................................................................................... iv Abstract .......................................................................................................................... v Introduction ................................................................................................................... 1 Analysis of Synthetic Mixture of 18 Sugars ......................................................... 9 Rat Urine Analysis .............................................................................................. 14 Cellular Permeability of Hyaluronic Acid Disaccharide ...................................... 20 Results and Discussion .............................................................................................. 22 Analysis of Synthetic Mixture of 18 Sugars ....................................................... 23 Rat Urine Analysis -

Pyrroles and Its Analogues to Conjugated Materials A
APPLICATIONS OF DITHIENO[3,2-b:2’,3’-d]PYRROLES AND ITS ANALOGUES TO CONJUGATED MATERIALS A Thesis Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By Casey Brian McCausland In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Major Department: Chemistry and Biochemistry June 2014 Fargo, North Dakota North Dakota State University Graduate School Title Applications of Dithieno[3,2-b:2',3'-d]pyrroles and its Analogues to Conjugated Materials By Casey Brian McCausland The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of MASTER OF SCIENCE SUPERVISORY COMMITTEE: Seth C. Rasmussen Chair Andrew Croll Erika Offerdahl Pinjing Zhao Approved: 6/20/2014 Gregory R. Cook Date Department Chair ABSTRACT The promise of semiconducting materials with tunable electronic and optical properties that share the same mechanical flexibility, low production costs and ease of processing displayed by traditional polymers fuels the intense interest seen in developing devices employing conjugated polymers (CPs). Many examples of -conjugated systems have been studied and reported in the literature, of which Rasmussen and coworkers have advanced the field with their work involving N-alkyl, N-aryl, and N-acyl-dithieno[3,2-b:2',3'-d]pyrroles (DTPs). Using DTP as a template current investigations are targeting two analogues: pyrrolo[3,2-d:4,5-d’]bisthiazole (PBTz) and difuro[3,2-b:2’,3’-d]pyrroles (DFP). In comparison to polymers of DTP, the electron-deficient nature of thiazoles is known to stabilize HOMO levels of PBTz-based polymers. -

United States Patent (10) Patent No.: US 7,118,616 B2 Gillard Et Al
USOO711 8616B2 (12) United States Patent (10) Patent No.: US 7,118,616 B2 Gillard et al. (45) Date of Patent: Oct. 10, 2006 (54) PAINT COMPOSITIONS COMPRISING 5,382.281 A 1, 1995 Kuo ESTERS OF ROSIN AND PROCESS OF 5,436,284 A 7/1995 Honda et al. PRODUCTION THEREOF 5,545,823. A 8, 1996 Kuo et al. 5,712.275 A 1/1998 Van Gestel (75) Inventors: Michel Gillard, Louvain-la-Neuve 5,767,171 A 6/1998 MatSubara et al. (BE); M Vos, Nivelles (BE) 5,795,374. A * 8/1998 Itoh et al. ..................... 106/16 ; Varcel Vos, IN1Velles 6,069,189 A 5/2000 Kramer et al. 6,110,990 A 8/2000 Nakamura et al. (73) Assignee: Sigma Coatings B.V., Uithoorn (NL) 6,248,806 B1 6/2001 Codolar et al. 6,303,701 B1 10/2001 Isozaki et al. (*) Notice: Subject to any disclaimer, the term of this 6,559,202 B1 5/2003 t al patent is extended or adjusted under 35 6,710, 117 B1 3/2004 Gillard et al. U.S.C. 154(b) by 0 days. 6,828,030 B1* 12/2004 Arimura et al. ............ 428.447 2003,0162924 A1 8, 2003 Vos et al. (21) Appl. No.: 10/492,766 2005/008O159 A1 4/2005 Omoto et al. ............... 523,122 (22) PCT Filed: Oct.e 25,a? a 9 2002 FOREIGN PATENT DOCUMENTS EP OOO1711 * 5, 1979 (86). PCT No.: PCT/EPO2/11957 EP O 131 621 9, 1987 EP O 297 505 1, 1989 S 371 (c)(1), EP O 526 441 2, 1993 (2), (4) Date: Feb. -

Methyl(Bismethylthio)Sulphonium Salts and Organo-Sulphur Chemistry
Pure & App!. Chem., Vol. 59, No. 8, pp. 989—992, 1987. Printed in Great Britain. © 1987 IUPAC Methyl(bismethylthio)sulphonium salts and trimethylsilyl-sulphenyl halides as synthons in organo-sulphur chemistry GiuseppeCapozzi Department of Organic Chemistry, University of Florence, 50121 Florence, Italy Abstract —Methyl(bismethylthio)sulfoniumsalts 1 react with nucleophiles like suiphides, alkenes, and alkynes to give respectively methyltiosulphonium, thiiranium, and thiirenium salts. The reaction of 1 with alkenes or alkynes hearing a nucleophilic center inappropriatepositiongives ring closure with formation of heterocyclic compounds whose nature depend on the position of the nucleophile in the unsaturated hydrocarbons. A new one—pot synthesis of thiiranes from alkenes and trimethylsilylsulphenyl bromide is also described. The timethylsilylsulphenyl bromide is also the key intermediate for a new catalytic desulphurization of thiiranes to alkenes by trimethylsi lyl bromide. Several years ago we synthesized some methyl(bismethylthio)sulphonium salts 1 and found that they may act as very efficient synthetic equivalents of the rrethylsulphenylium ion in reactions with nucleophiles of various nature1' +S-Me - + Scheme! Me—S' X Me—S S-Me +,S—Me + A R______ + Me—S—S-Me 2 Me_SSM + S Me—S—-.S( X — SbC1. BF4 1 2 Methyl(bi smethylt hi o) sulphoni um hex a c h lo roan t imona te react s w it h sulphidesto give methylthio—substituted sulphonium salts 2 (Scheme 1). The reactions were carried out with symmetric and asymmetric sulphides as well as with cyclic sulphides. Usually the thio—substituted sulphonium salts are prepared by alkylation of disulphides; however the alkylation of an asymmetric disulphide gives poor regioselectivity and the synthesis of cyclic thio—substituted sulphonium salts is not possible because of the unavailability of the corresponding disulphides. -

ION/MOLECULE REACTIONS STUDIED with the FLOWING AFTERGLOW and THEORETICAL METHODOLOGY by Kyle Richard Tilger B. A., Washington A
ION/MOLECULE REACTIONS STUDIED WITH THE FLOWING AFTERGLOW AND THEORETICAL METHODOLOGY by Kyle Richard Tilger B. A., Washington and Jefferson College, 2002 Submitted to the Graduate Faculty of Arts and Sciences in partial fulfillment of the requirements for the degree of Master of Science University of Pittsburgh 2006 UNIVERSITY OF PITTSBURGH FACULTY OF ARTS AND SCIENCES This thesis was presented by Kyle Richard Tilger It was defended on April 21, 2006 and approved by Kenneth D. Jordan Peter E. Siska Thesis Advisor: Dr. Joseph J. Grabowski ii Copyright © by Kyle Richard Tilger 2006 iii ION/MOLECULE REACTIONS STUDIED WITH THE FLOWING AFTERGLOW AND THEORETICAL METHODOLOGY Kyle Richard Tilger, M.S. University of Pittsburgh, 2006 Initial interest in ion/molecule chemistry was because of its importance in atmospheric chemistry, but researchers have moved to investigating other interests involving organic reaction mechanisms. The study of ion/molecule reactions has flourished with the invention of instruments, such as the flowing afterglow instrument, and the advances in computational chemistry. The hydronium ion has been utilized as a reagent ion in ion/molecule chemistry for the detection of volatile organic compounds (VOCs). A limitation to the utility of the hydronium ion is that it is susceptible to clustering reactions with water. The propensity to cluster can be eliminated by replacing the hydrogens on the hydronium ion with trimethylsilyl groups to form + the tris-trimethylsilyloxonium ion, (TMS)3O . This strategy takes advantage of the trimethylsilyl cation’s proclivity to react as if it were a proton. + + + Hexamethyldisiloxane was allowed to react with TMS , (TMS)2Cl , TMSOH2 , and + + TMSC6H6 in an attempt to form (TMS)3O . -

Synthesis of Peropyrene and Tetracene Derivatives For
SYNTHESIS OF PEROPYRENE AND TETRACENE DERIVATIVES FOR PHOTOCHEMICAL APPLICATIONS Marco Tulio Rodríguez López, L. C. Q. Dissertation Prepared for the Degree of DOCTOR OF PHILOSOPHY UNIVERSITY OF NORTH TEXAS May 2015 APROVED: W. Justin Youngblood, Committee Chair Sushama A. Dandekar, Committee Member Mohammad A. Omary, Committee Member Robby A. Petros, Committee Member William E. Acree, Jr. Chair of the Department of Chemistry Mark Wardell, Dean of the Toulouse Graduate School Rodríguez López, Marco Tulio. Synthesis of Peropyrene and Tetracene Derivatives for Photochemical Applications. Doctor of Philosophy (Chemistry-Organic Chemistry), May 2015, 120 pp., 6 tables, 105 figures, 48 schemes, 78 numbered references. A novel route for the synthesis of the polycyclic aromatic hydrocarbon peropyrene (Pp) is reported along with the efforts to synthesize derivatives of Pp, 2,2′- and 5,5′-linked tetracene dimers as candidates for study as singlet fission materials in photovoltaic devices. Peropyrene was synthesized by the McMurry coupling conditions from phenalenone and low-valent titanium species. The crystal structure of Pp is formed by π-stacked molecular pairs in a herringbone arrangement. The direct functionalization of Pp was studied, and several indirect methods for the functionalization of Pp via phenalenone derivatives are reported. Nucleophilicly dependent, regioselective Michael addition pathways for phenalenone are described. Phenalenone forms a nucleophilic complex with bispinacolatodiboron and yields chiral 3,3′-linked phenalenone dimers and a bicyclo[3.2.1]octane derivative product of an unusual 3,4 addition. An active complex product of phenalenone and (dimethylphenylsilyl)boronic acid pinacolic ester forms Pp directly. The synthesis of 2,2′- and 5,5′-linked tetracene dimers led to the study of the reduction of 1-arylprop-2-yn-1-ol derivatives via TFA-catalyzed hydride transfer from triethylsilane. -

Transcription 12.02.10A
Lecture 10A • 02/10/12 Protecting groups I want to show you another common protecting group used for alcohols. It’s the formation of what are known as silyl ethers. The version that your text has is the TMS ether. Don’t get that confused with tetramethylsilane, which is our standard that we use in NMR; this is trimethylsilyl; this is very close in form. What it is – trimethylsilyl chloride is the reagent that’s generally used. When you react it with an alcohol, the silicon-chlorine bond is active enough that you’re able to do an Sn2-style reaction, even is you just have the neutral alcohol. You might notice that there’s a whole bunch of alkyl groups on the silicon; how can you do an Sn2 with something that’s got that much steric hinderance? Silicon’s larger enough that you don’t have the same form of steric crowding, so this reaction is able to take place. We have the regular form of deprotonation step afterwards. If it weren’t for the fact that we have a silicon there instead of a carbon, this would just be a regular old ether, but that’s why this is called a silyl ether. That somes from the fact that SiH4 is called silane. The -ane is actually used for other atoms besides carbon. You can have phosphane, silane – usually means add hydrogens to that particular element. Silicon can take four just like carbon because it’s in the same column of the periodic table as carbon. This particular protecting group is very widely used, but it has certain drawbacks, in that it very easily reacts in both acidic and basic media. -

Ozonolysis of Silyl Enol Ethers: Synthesis of 3-Silyloxy-1,2-And 3-Alkyl-3-Silyloxy-1,2-Dioxolanes Katrina Wilson
University of Redlands InSPIRe @ Redlands Undergraduate Honors Theses Theses, Dissertations, and Honors Projects 2015 Ozonolysis of silyl enol ethers: Synthesis of 3-silyloxy-1,2-and 3-alkyl-3-silyloxy-1,2-dioxolanes Katrina Wilson Follow this and additional works at: https://inspire.redlands.edu/cas_honors Part of the Medicinal-Pharmaceutical Chemistry Commons, Organic Chemistry Commons, and the Other Chemistry Commons Recommended Citation Wilson, K. (2015). Ozonolysis of silyl enol ethers: Synthesis of 3-silyloxy-1,2-and 3-alkyl-3-silyloxy-1,2-dioxolanes (Undergraduate honors thesis, University of Redlands). Retrieved from https://inspire.redlands.edu/cas_honors/123 This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 License This material may be protected by copyright law (Title 17 U.S. Code). This Open Access is brought to you for free and open access by the Theses, Dissertations, and Honors Projects at InSPIRe @ Redlands. It has been accepted for inclusion in Undergraduate Honors Theses by an authorized administrator of InSPIRe @ Redlands. For more information, please contact [email protected]. Ozonolysis of silyl enol ethers: Synthesis of 3-silyloxy-1,2- and 3-alkyl-3-silyloxy-1,2-dioxolanes By: Katrina Wilson A thesis submitted to the Faculty of the Department of Chemistry at The University of Redlands in partial fulfillment of the requirements for a Bachelor of Science degree Department of Chemistry 2015 ii Abstract Katrina Wilson (B.S. Chemistry and Biology) Synthesis and Ozonolysis of Silyl Enol Ethers Thesis Directed by Dr. David Soulsby Silyl enol ethers are important synthetic intermediates that are used in a variety of reactions. -

Supporting Information a Combination of Trimethylsilyl Chloride and Hydrous Natural Montmorillonite Clay
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2015 Supporting Information A Combination of Trimethylsilyl chloride and hydrous natural montmorillonite clay: An efficient solid acid catalyst for the azidation of benzylic and allylic alcohols with trimethylsilyl azide Michael A. Tandiary,a Yoichi Masuib and Makoto Onakab* aDepartment of Chemistry, Graduate School of Sciences, The University of Tokyo, Bunkyo-Ku Hongo 7-3-1, Tokyo 113-0033, Japan bDepartment of Multi-Disciplinary Sciences, Graduate School of Arts and Sciences, The University of Tokyo, Meguro-Ku Komaba 3-8-1, Tokyo 153-8902, Japan I. General remark All solvents and triethylamine were obtained from Kanto Chemical and used without further purification. Alcohols 1a, 1b, 1c, 1d, 1e, 1i, 1j, 1k, 1l, 1m, 1n, 1o, Me3SiCl, Me3SiN3 and propionic acid were obtained from Tokyo Chemical Industry (TCI), Japan. Alcohols 1f and 1g were prepared by reduction of the corresponding carbonyl compounds (ketones or aldehydes) with LiAlH4 in anhydrous diethyl ether. Alcohols 1h was prepared by reduction of the corresponding carbonyl compounds (conjugated ketones) with NaBH4 in methanol. Kunipia-F (Na, 2.69; Al, 11.8; Fe, 1.46; Mg, 1.97%. Cation-exchange capacity = 1.19 mequiv g-1) was obtained from Kunimine Industry, Japan, and used as hydrous Na-Mont containing 14wt% of water. CuI was obtained from Wako Pure Chemical Industries. Phenyl acetylene was obtained from Sigma-Aldrich. Commercially available CH2Cl2 (Kanto Chemical Inc.) was used without drying treatment: water content=<0.2% according to the supplier’s specification data: https:cica-web.kanto.co.jp/CicaWeb/spec/E_EPDCM11.pdf).