A Novel Isoform of Cryptochrome 4 (Cry4b)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

American Robin
American Robin DuPage Birding Club, 2020 American Robin Appearance A chunky, heavy-bodied bird with a relatively small dark head. Sexually dimorphic, meaning the male and female look different. American Robins are a uniform dark gray with a brick red breast. Female Male Females are a lighter gray with a lighter breast. Males tend to be darker with a brighter red breast. Males are larger than females. Photos: Elmarie Von Rooyen (left), Jackie Tilles (right) DuPage Birding Club, 2020 2 American Robin Appearance American Robins are a medium-size bird with a length of about ten inches. They are so common that they are a good bird to compare size with when you come across an unknown bird. Is the bird bigger than an American Robin or smaller than an American Robin? Judging the size of a bird is very helpful in identifying an unknown bird. Chart: The Cornell Lab, All About Birds https://www.allaboutbirds.org/guide/American_Robin/id DuPage Birding Club, 2020 3 American Robin Appearance Juvenile American Robins have a speckled breast with a tint of rusty red. Photos: Natalie McFaul DuPage Birding Club, 2020 4 American Robin Sounds From The Cornell Lab of Ornithology: https://www.birds.cornell.edu/home/ SONGS The musical song of the American Robin is a familiar sound of spring. It’s a string of 10 or so clear whistles assembled from a few often- repeated syllables, and often described as cheerily, cheer up, cheer up, cheerily, cheer up. The syllables rise and fall in pitch but are delivered at a steady rhythm, with a pause before the bird begins singing again. -

Dieter Thomas Tietze Editor How They Arise, Modify and Vanish
Fascinating Life Sciences Dieter Thomas Tietze Editor Bird Species How They Arise, Modify and Vanish Fascinating Life Sciences This interdisciplinary series brings together the most essential and captivating topics in the life sciences. They range from the plant sciences to zoology, from the microbiome to macrobiome, and from basic biology to biotechnology. The series not only highlights fascinating research; it also discusses major challenges associated with the life sciences and related disciplines and outlines future research directions. Individual volumes provide in-depth information, are richly illustrated with photographs, illustrations, and maps, and feature suggestions for further reading or glossaries where appropriate. Interested researchers in all areas of the life sciences, as well as biology enthusiasts, will find the series’ interdisciplinary focus and highly readable volumes especially appealing. More information about this series at http://www.springer.com/series/15408 Dieter Thomas Tietze Editor Bird Species How They Arise, Modify and Vanish Editor Dieter Thomas Tietze Natural History Museum Basel Basel, Switzerland ISSN 2509-6745 ISSN 2509-6753 (electronic) Fascinating Life Sciences ISBN 978-3-319-91688-0 ISBN 978-3-319-91689-7 (eBook) https://doi.org/10.1007/978-3-319-91689-7 Library of Congress Control Number: 2018948152 © The Editor(s) (if applicable) and The Author(s) 2018. This book is an open access publication. Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. -

Bird Checklists of the World Country Or Region: Ghana
Avibase Page 1of 24 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Ghana 2 Number of species: 773 3 Number of endemics: 0 4 Number of breeding endemics: 0 5 Number of globally threatened species: 26 6 Number of extinct species: 0 7 Number of introduced species: 1 8 Date last reviewed: 2019-11-10 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Ghana. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=gh [26/09/2021]. Make your observations count! Submit your data to ebird. -

Progress in the Development of an Eurasian-African Bird Migration Atlas
CONVENTION ON UNEP/CMS/COP13/Inf.20 MIGRATORY 10 February 2020 SPECIES Original: English 13th MEETING OF THE CONFERENCE OF THE PARTIES Gandhinagar, India, 17 - 22 February 2020 Agenda Item 25 PROGRESS IN THE DEVELOPMENT OF AN EURASIAN-AFRICAN BIRD MIGRATION ATLAS (Submitted by the European Union of Bird Ringing (EURING) and the Institute of Avian Research) Summary: The African-Eurasian Bird Migration Atlas is being developed under the auspices of CMS in the framework of a Global Animal Migration Atlas, of which it constitutes a module. The African-Eurasian Bird Migration Atlas is being developed and compiled by the European Union of Bird Ringing (EURING) under a Project Cooperation Agreement (PCA) between the CMS Secretariat and the Institute of Avian Research, acting on behalf of EURING. The development of the African-Eurasian Bird Migration Atlas is funded with the contribution granted by the Government of Italy under the Migratory Species Champion Programme. This information document includes a progress report on the development of the various components of the project. The project is expected to be completed in 2021. UNEP/CMS/COP13/Inf.20 Eurasian-African Bird Migration Atlas progress report February 2020 Stephen Baillie1, Franz Bairlein2, Wolfgang Fiedler3, Fernando Spina4, Kasper Thorup5, Sam Franks1, Dorian Moss1, Justin Walker1, Daniel Higgins1, Roberto Ambrosini6, Niccolò Fattorini6, Juan Arizaga7, Maite Laso7, Frédéric Jiguet8, Boris Nikolov9, Henk van der Jeugd10, Andy Musgrove1, Mark Hammond1 and William Skellorn1. A report to the Convention on Migratory Species from the European Union for Bird Ringing (EURING) and the Institite of Avian Research, Wilhelmshaven, Germany 1. British Trust for Ornithology, Thetford, IP24 2PU, UK 2. -

Territoriality As a Paternity Guard in the European Robin, Erithacus Rubecula
ANIMAL BEHAVIOUR, 2000, 60, 165–173 doi:10.1006/anbe.2000.1442, available online at http://www.idealibrary.com on ARTICLES Territoriality as a paternity guard in the European robin, Erithacus rubecula JOE TOBIAS & NAT SEDDON Department of Zoology, University of Cambridge (Received 29 June 1999; initial acceptance 29 July 1999; final acceptance 18 March 2000; MS. number: 6273) To investigate the relative importance of paternity defences in the European robin we used behavioural observations, simulated intrusions and temporary male removal experiments. Given that paired males did not increase their mate attendance, copulation rate or territory size during the female’s fertile period, the most frequently quoted paternity assurance strategies in birds were absent. However, males with fertile females sang and patrolled their territories more regularly, suggesting that territorial motivation and vigilance were elevated when the risk of cuckoldry was greatest. In addition, there was a significant effect of breeding period on response to simulated intrusions: residents approached and attacked freeze-dried mounts more readily in the fertile period. During 90-min removals of the pair male in the fertile period, neighbours trespassed more frequently relative to prefertile and fertile period controls and appeared to seek copulations with unattended females. When replaced on their territories, males immediately increased both song rate and patrolling rate in comparison with controls. We propose that male robins sing to signal their presence, and increase their territorial vigilance and aggression in the fertile period to protect paternity. 2000 The Association for the Study of Animal Behaviour Despite the apparent social monogamy of the majority of Thryothorus nigricapillus: Levin 1996). -
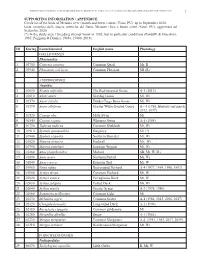
1 ID Euring Latin Binomial English Name Phenology Galliformes
BIRDS OF METAURO RIVER: A GREAT ORNITHOLOGICAL DIVERSITY IN A SMALL ITALIAN URBANIZING BIOTOPE, REQUIRING GREATER PROTECTION 1 SUPPORTING INFORMATION / APPENDICE Check list of the birds of Metauro river (mouth and lower course / Fano, PU), up to September 2020. Lista completa delle specie ornitiche del fiume Metauro (foce e basso corso /Fano, PU), aggiornata ad Settembre 2020. (*) In the study area 1 breeding attempt know in 1985, but in particolar conditions (Pandolfi & Giacchini, 1985; Poggiani & Dionisi, 1988a, 1988b, 2019). ID Euring Latin binomial English name Phenology GALLIFORMES Phasianidae 1 03700 Coturnix coturnix Common Quail Mr, B 2 03940 Phasianus colchicus Common Pheasant SB (R) ANSERIFORMES Anatidae 3 01690 Branta ruficollis The Red-breasted Goose A-1 (2012) 4 01610 Anser anser Greylag Goose Mi, Wi 5 01570 Anser fabalis Tundra/Taiga Bean Goose Mi, Wi 6 01590 Anser albifrons Greater White-fronted Goose A – 4 (1986, february and march 2012, 2017) 7 01520 Cygnus olor Mute Swan Mi 8 01540 Cygnus cygnus Whooper Swan A-1 (1984) 9 01730 Tadorna tadorna Common Shelduck Mr, Wi 10 01910 Spatula querquedula Garganey Mr (*) 11 01940 Spatula clypeata Northern Shoveler Mr, Wi 12 01820 Mareca strepera Gadwall Mr, Wi 13 01790 Mareca penelope Eurasian Wigeon Mr, Wi 14 01860 Anas platyrhynchos Mallard SB, Mr, W (R) 15 01890 Anas acuta Northern Pintail Mi, Wi 16 01840 Anas crecca Eurasian Teal Mr, W 17 01960 Netta rufina Red-crested Pochard A-4 (1977, 1994, 1996, 1997) 18 01980 Aythya ferina Common Pochard Mr, W 19 02020 Aythya nyroca Ferruginous -
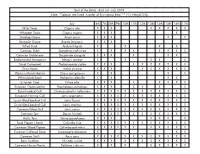
Best of the Baltic - Bird List - July 2019 Note: *Species Are Listed in Order of First Seeing Them ** H = Heard Only
Best of the Baltic - Bird List - July 2019 Note: *Species are listed in order of first seeing them ** H = Heard Only July 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th Mute Swan Cygnus olor X X X X X X X X Whopper Swan Cygnus cygnus X X X X Greylag Goose Anser anser X X X X X Barnacle Goose Branta leucopsis X X X Tufted Duck Aythya fuligula X X X X Common Eider Somateria mollissima X X X X X X X X Common Goldeneye Bucephala clangula X X X X X X Red-breasted Merganser Mergus serrator X X X X X Great Cormorant Phalacrocorax carbo X X X X X X X X X X Grey Heron Ardea cinerea X X X X X X X X X Western Marsh Harrier Circus aeruginosus X X X X White-tailed Eagle Haliaeetus albicilla X X X X Eurasian Coot Fulica atra X X X X X X X X Eurasian Oystercatcher Haematopus ostralegus X X X X X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X X X X X X X European Herring Gull Larus argentatus X X X X X X X X X X X X Lesser Black-backed Gull Larus fuscus X X X X X X X X X X X X Great Black-backed Gull Larus marinus X X X X X X X X X X X X Common/Mew Gull Larus canus X X X X X X X X X X X X Common Tern Sterna hirundo X X X X X X X X X X X X Arctic Tern Sterna paradisaea X X X X X X X Feral Pigeon ( Rock) Columba livia X X X X X X X X X X X X Common Wood Pigeon Columba palumbus X X X X X X X X X X X Eurasian Collared Dove Streptopelia decaocto X X X Common Swift Apus apus X X X X X X X X X X X X Barn Swallow Hirundo rustica X X X X X X X X X X X Common House Martin Delichon urbicum X X X X X X X X White Wagtail Motacilla alba X X -

Bird Number Dynamics During the Post-Breeding Period at the Tömörd Bird Ringing Station, Western Hungary
THE RING 39 (2017) 10.1515/ring-2017-0002 BIRD NUMBER DYNAMICS DURING THE POST-BREEDING PERIOD AT THE TÖMÖRD BIRD RINGING STATION, WESTERN HUNGARY József Gyurácz1*, Péter Bánhidi2, József Góczán2, Péter Illés2, Sándor Kalmár2, Péter Koszorús2, Zoltán Lukács1, Csaba Németh2, László Varga2 ABSTRACT Gyurácz J., Bánhidi P., Góczán J., Illés P., Kalmár S., Koszorús P., Lukács Z., Németh C. and Varga L. 2017. Bird number dynamics during the post-breeding period at the Tömörd Bird Ringing Station, western Hungary. Ring 39: 23-82. The fieldwork, i.e. catching and ringing birds using mist-nets, was conducted at Tömörd Bird Ringing Station in western Hungary during the post-breeding migration seasons in 1998-2016. Altogether, 106,480 individuals of 133 species were ringed at the station. The aim of this paper was to publish basic information on passerine migration at this site. Migration phenology was described through annual and daily capture frequencies. Further- more, we provide the median date of the passage, the date of the earliest or latest capture, the peak migration season within the study period, and the countries where the birds monitored at the site were ringed or recovered abroad. To compare the catching dynamics for the fifty species with total captures greater than 200, a reference period was defined: from 5 Aug. to 5 Nov. 2001-2016. Some non-passerines that are more easily caught with mist-nets or that are caught occasionally were listed as well. The two superdominant spe- cies, the European Robin and the Eurasian Blackcap, with 14,377 and 13,926 total cap- tures, made up 27% of all ringed individuals. -

GHANA MEGA Rockfowl & Upper Guinea Specials Th St 29 November to 21 December 2011 (23 Days)
GHANA MEGA Rockfowl & Upper Guinea Specials th st 29 November to 21 December 2011 (23 days) White-necked Rockfowl by Adam Riley Trip Report compiled by Tour Leader David Hoddinott RBT Ghana Mega Trip Report December 2011 2 Trip Summary Our record breaking trip total of 505 species in 23 days reflects the immense birding potential of this fabulous African nation. Whilst the focus of the tour was certainly the rich assemblage of Upper Guinea specialties, we did not neglect the interesting diversity of mammals. Participants were treated to an astonishing 9 Upper Guinea endemics and an array of near-endemics and rare, elusive, localized and stunning species. These included the secretive and rarely seen White-breasted Guineafowl, Ahanta Francolin, Hartlaub’s Duck, Black Stork, mantling Black Heron, Dwarf Bittern, Bat Hawk, Beaudouin’s Snake Eagle, Congo Serpent Eagle, the scarce Long-tailed Hawk, splendid Fox Kestrel, African Finfoot, Nkulengu Rail, African Crake, Forbes’s Plover, a vagrant American Golden Plover, the mesmerising Egyptian Plover, vagrant Buff-breasted Sandpiper, Four-banded Sandgrouse, Black-collared Lovebird, Great Blue Turaco, Black-throated Coucal, accipiter like Thick- billed and splendid Yellow-throated Cuckoos, Olive and Dusky Long-tailed Cuckoos (amongst 16 cuckoo species!), Fraser’s and Akun Eagle-Owls, Rufous Fishing Owl, Red-chested Owlet, Black- shouldered, Plain and Standard-winged Nightjars, Black Spinetail, Bates’s Swift, Narina Trogon, Blue-bellied Roller, Chocolate-backed and White-bellied Kingfishers, Blue-moustached, -

Niche Analysis and Conservation of Bird Species Using Urban Core Areas
sustainability Article Niche Analysis and Conservation of Bird Species Using Urban Core Areas Vasilios Liordos 1,* , Jukka Jokimäki 2 , Marja-Liisa Kaisanlahti-Jokimäki 2, Evangelos Valsamidis 1 and Vasileios J. Kontsiotis 1 1 Department of Forest and Natural Environment Sciences, International Hellenic University, 66100 Drama, Greece; [email protected] (E.V.); [email protected] (V.J.K.) 2 Arctic Centre, University of Lapland, 96101 Rovaniemi, Finland; jukka.jokimaki@ulapland.fi (J.J.); marja-liisa.kaisanlahti@ulapland.fi (M.-L.K.-J.) * Correspondence: [email protected] Abstract: Knowing the ecological requirements of bird species is essential for their successful con- servation. We studied the niche characteristics of birds in managed small-sized green spaces in the urban core areas of southern (Kavala, Greece) and northern Europe (Rovaniemi, Finland), during the breeding season, based on a set of 16 environmental variables and using Outlying Mean Index, a multivariate ordination technique. Overall, 26 bird species in Kavala and 15 in Rovaniemi were recorded in more than 5% of the green spaces and were used in detailed analyses. In both areas, bird species occupied different niches of varying marginality and breadth, indicating varying responses to urban environmental conditions. Birds showed high specialization in niche position, with 12 species in Kavala (46.2%) and six species in Rovaniemi (40.0%) having marginal niches. Niche breadth was narrower in Rovaniemi than in Kavala. Species in both communities were more strongly associated either with large green spaces located further away from the city center and having a high vegetation cover (urban adapters; e.g., Common Chaffinch (Fringilla coelebs), European Greenfinch (Chloris Citation: Liordos, V.; Jokimäki, J.; chloris Cyanistes caeruleus Kaisanlahti-Jokimäki, M.-L.; ), Eurasian Blue Tit ( )) or with green spaces located closer to the city center Valsamidis, E.; Kontsiotis, V.J. -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Notices
21262 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Notices acquisition were not included in the 5275 Leesburg Pike, Falls Church, VA Comment (1): We received one calculation for TDC, the TDC limit would not 22041–3803; (703) 358–2376. comment from the Western Energy have exceeded amongst other items. SUPPLEMENTARY INFORMATION: Alliance, which requested that we Contact: Robert E. Mulderig, Deputy include European starling (Sturnus Assistant Secretary, Office of Public Housing What is the purpose of this notice? vulgaris) and house sparrow (Passer Investments, Office of Public and Indian Housing, Department of Housing and Urban The purpose of this notice is to domesticus) on the list of bird species Development, 451 Seventh Street SW, Room provide the public an updated list of not protected by the MBTA. 4130, Washington, DC 20410, telephone (202) ‘‘all nonnative, human-introduced bird Response: The draft list of nonnative, 402–4780. species to which the Migratory Bird human-introduced species was [FR Doc. 2020–08052 Filed 4–15–20; 8:45 am]‘ Treaty Act (16 U.S.C. 703 et seq.) does restricted to species belonging to biological families of migratory birds BILLING CODE 4210–67–P not apply,’’ as described in the MBTRA of 2004 (Division E, Title I, Sec. 143 of covered under any of the migratory bird the Consolidated Appropriations Act, treaties with Great Britain (for Canada), Mexico, Russia, or Japan. We excluded DEPARTMENT OF THE INTERIOR 2005; Pub. L. 108–447). The MBTRA states that ‘‘[a]s necessary, the Secretary species not occurring in biological Fish and Wildlife Service may update and publish the list of families included in the treaties from species exempted from protection of the the draft list. -

Tape Lures Swell Bycatch on a Mediterranean Island Harbouring Illegal Bird Trapping
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.13.991034; this version posted March 15, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Tape lures swell bycatch on a Mediterranean island 2 harbouring illegal bird trapping 3 Matteo Sebastianelli1, Georgios Savva1, Michaella Moysi1, and 4 Alexander N. G. Kirschel1* 5 1Department of Biological Sciences, University of Cyprus, PO Box 20537, Nicosia 1678, 6 Cyprus 7 8 Keywords: Cyprus, illegal bird hunting, Sylvia atricapilla, Sylvia melanocephala, playback 9 experiment, warbler. 10 *Email of corresponding author: [email protected] 11 12 bioRxiv preprint doi: https://doi.org/10.1101/2020.03.13.991034; this version posted March 15, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 13 Abstract 14 Mediterranean islands are critical for migrating birds, providing shelter and sustenance 15 for millions of individuals each year. Humans have long exploited bird migration 16 through hunting and illegal trapping. On the island of Cyprus, trapping birds during 17 their migratory peak is considered a local tradition, but has long been against the law. 18 Illegal bird trapping is a lucrative business, however, with trappers using tape lures that 19 broadcast species’ vocalizations because it is expected to increase numbers of target 20 species.