INDUCTION of RESISTANCE to PYRICULARIYA ORYZAE in UNDERLOWLAND RICE ECOSYSTEM by SILICATE SOLUBILIZING BACTERIA Dr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tamil Nadu Public Service Commission Bulletin
© [Regd. No. TN/CCN-466/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 196/2009 2017 [Price: Rs. 156.00 Paise. TAMIL NADU PUBLIC SERVICE COMMISSION BULLETIN No. 7] CHENNAI, THURSDAY, MARCH 16, 2017 Panguni 3, Thunmugi, Thiruvalluvar Aandu-2048 CONTENTS DEPARTMENTAL TESTS—RESULTS, DECEMBER 2016 Name of the Tests and Code Numbers Pages Pages Departmental Test For officers of The Co-operative Departmental Test For Members of The Tamil Nadu Department - Co-operation - First Paper (Without Ministerial Service In The National Employment Books) (Test Code No. 003) .. 627-631 Service (Without Books)(Test Code No. 006) .. 727 Departmental Test For officers of The Co-operative The Jail Test - Part I - (A) The Indian Penal Code (With Department - Co-operation - Second Paper (Without Books) (Test Code No. 136) .. .. 728-729 Books) (Test Code No. 016) .. .. 632-636 Departmental Test For officers of The Co-operative The Jail Test - Part I - (B) The Code of Criminal 729-730 Department - Auditing - First Paper (Without Procedure (With Books) (Test Code No. 154) .. Books)(Test Code No. 029) .. .. 636-641 The Jail Test - Part Ii -- Juvenile Justice (Care And Departmental Test For officers of The Co-operative Protection.. of Children) Act, 2000 (Central Act 56 of Department - Auditing - Second Paper (Without 2000).. (With Books) (Test Code No. 194) .. 730 Books)(Test Code No. 044) .. 641-645 The Jail Test -- Part I -- (C) Laws, Rules, Regulations Departmental Test For officers of The Co-operative And Orders Relating To Jail Management (With Department - Banking (Without Books) (Test Code Books)(Test Code No. 177) .. .. 731-732 No. -

Reservations of Offices
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2016 [Price: Rs. 71.20 Paise. TAMIL NADU GOVERNMENT GAZETTE EXTRAORDINARY PUBLISHED BY AUTHORITY No. 209] CHENNAI, FRIDAY, SEPTEMBER 16, 2016 Aavani 31, Thunmugi, Thiruvalluvar Aandu–2047 Part II—Section 2 Notifications or Orders of interest to a section of the public issued by Secretariat Departments. NOTIFICATIONS BY GOVERNMENT RURAL DEVELOPMENT AND PANCHAYAT RAJ DEPARTMENT RESERVATION OF OFFICES OF THE CHAIRMEN OF DISTRICT PANCHAYATS FOR THE PERSONS BELONGING TO THE SCHEDULED CASTES AND SCHEDULED TRIBES AND FOR WOMEN UNDER THE TAMIL NADU PANCHAYATS ACT, 1994. [G.O. (Ms.) No. 102, Rural Development and Panchayat Raj (PR-1) Department, 16th September 2016, ÝõE 31, ¶¡ºA, F¼õœÀõ˜ ݇´-2047.] No. II(2)/RDPR/640(a-1)/2016 Under Section 57 of the Tamil Nadu Panchayats Act, 1994 (Tamil Nadu Act 21 of 1994), the Governor of Tamil Nadu hereby reserves the offices of the Chairmen of District Panchayats for the persons belonging to the Scheduled Castes and Scheduled Tribes and for Women as specified in the table below:- II-2 Ex. (209) 2 TAMIL NADU GOVERNMENT GAZETTE EXTRAORDINARY THE TABLE RESERVATION OF OFFICES OF CHAIRMEN OF DISTRICT PANCHAYATS Sl. Category to which reservation is Name of the District No. made (1) (2) (3) 1 The Nilgiris ST General 2 Namakkal SC Women 3 Tiruppur SC Women 4 Virudhunagar SC Women 5 Tirunelveli SC Women 6 Thanjavur SC General 7 Ariyalur SC General 8 Dindigul SC General 9 Ramanathapuram SC General 10 Kancheepuram General Women 11 Tiruvannamalai -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2009-11. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2010 [Price: Rs. 23.20 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 27] CHENNAI, WEDNESDAY, JULY 14, 2010 Aani 30, Thiruvalluvar Aandu–2041 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 1259-1316 Notice .. 1316 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES My son, P. Manoj, born on 8th October 1996 (native My daughter, R. Harini, daughter of Thiru A.S. Ranganathan, district: Erode), residing at Old No. 2/26, New No. 1/79, born on 15th December 1993 (native district: Thiruvannamalai), Kongampalayam, Chittode, Erode-638 102, shall hencefroth residing at No. 122, Bharathi Street, V.G.P. Shanthi Nagar, be known as P. METHUNRAJ. Narayanapuram, Chennai-600 100, shall henceforth be known as R. SRIHARINI. K.R.E. PONGI. Chittode, 5th July 2010. (Father.) RAJALAKSHMI RANGAN. Chennai, 5th July 2010. (Mother.) My son, P. Yaswanth, born on 14th October 1999 (native I, M.C. Deepa, wife of Thiru Bhuvanesh Srinivasan, born district: Erode), residing at Old No. 2/26, New No. 1/79, on 21st May 1977 (native district: Kancheepuram), residing Kongampalayam, Chittode, Erode-638 102, shall henceforth at Old No. 85, New No. 34, Gengu Reddy Street, be known as K.E.P. -

2 Half Yearly Monitoring Report on Mid Day Meal Scheme for the State of Tamil Nadu
2nd HALF YEARLY MONITORING REPORT ON MID DAY MEAL SCHEME FOR THE STATE OF TAMIL NADU SUBMITTED BY INDIAN INSTITUTE OF TECHNOLOGY MADRAS Period: 1st October 2013 to 31st March 2014 Districts Covered 1. THANJAVUR.................................................................... 3 - 22 2. THIRUVARUR.................................................................. 23-44 3. NAGAPATTINAM..............................................................45-64 1 2ND HALF YEARLY MONITORING REPORT ON MID DAY MEAL SCHEME IN THE STATE OF TAMIL NADU FOR THE PERIOD October 2013 TO March 2014 INTRODUCTION The Monitoring of Mid-day Meal Scheme in 13 districts of Tamil Nadu is being carried out by Indian Institute of Technology Madras (IITM) as a 3rd party evaluating institute with the guidance and support of MHRD. In the 1st Phase, IITM has conducted the monitoring and evaluation in 3 districts, Thanjavur, Thiruvarur and Nagapattinam. The tool for data collection has been prepared and given by MHRD. The selection of schools for monitoring was coordinated by the district SSA office and the monitoring carried out with the support of District Collector Office of the respective districts. Accordingly, the Monitoring Institute selected 40 schools from each district as per the guidelines provided by MHRD. This monitoring report has been prepared based on the school visits, interviews with HM, teachers, SMC members, MDM organiser, Cook/Helpers and discussions with the students and community. 2 THANJAVUR DISTRICT 1. At school level S.No. Indicators Source of Information 1. Availability of food grains School level i) Whether buffer stock of food grains for one month is available at registers, the school? MDM The buffer stock is maintained in all the 40 schools for 45 days in Thanjavur Registers, district. -
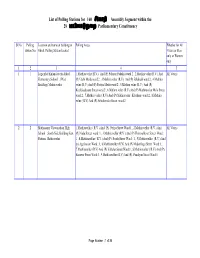
List of Polling Stations for 160 சீர்காழி Assembly Segment Within the 28 மய�லா��ைற Parliamentary Constituency
List of Polling Stations for 160 சீர்காழி Assembly Segment within the 28 மயலாைற Parliamentary Constituency Sl.No Polling Location and name of building in Polling Areas Whether for All station No. which Polling Station located Voters or Men only or Women only 12 3 4 5 1 1 Logambal Kulandaivelu Aided 1.Mathira velur (R.V.) And (P) Paluran Padukai ward 2 , 2.Mathira velur (R.V.) And All Voters Elementary School ,(West (P) Utchi Medu ward:2 , 3.Mathira velur (R.V) And (P) Ethakudi ward:2 , 4.Mathira Building) Mathiravelur velur (R.V) And (P) Pattiya Medu ward:2 , 5.Mathira velur (R.V) And (P) Keelakudiyana Street ward:2 , 6.Mathira velur (R.V) And (P) Mathiravelur Mela Street ward:2 , 7.Mathira velur ( R.V) And (P) Mathiravelur Kilatheru ward:2 , 8.Mathira velur ( R.V) And (P) Athidravidar Street ward:2 2 2 Muthusamy Viswanathan High 1.Mathiravellur ( R.V ) And (P) Periya Street Ward:1 , 2.Mathiravellur (R.V.) And All Voters School ,South Side Building East (P) Nadu Street ward :1 , 3.Mathiravellur (R.V ) And (P) Thiruvalluvar Street Ward : Portion, Mathiravelur 1 , 4.Mathiravellur ( R.V.) And (P) South Street Ward : 1 , 5.Mathiravellur (R.V.) And (p) Agraharam Ward :1 , 6.Mathiravellur (R.V) And (P) Madavilaga Street Ward:1 , 7.Mathiravellur (R.V) And (P) Vallalar Street Ward:1 , 8.Mathiravellur ( R.V) And (P) Kuravar Street Ward:1 , 9.Mathiravellur (R.V) And (P), Pandiyan Street Ward:1 Page Number : 1 of 84 List of Polling Stations for 160 சீர்காழி Assembly Segment within the 28 மயலாைற Parliamentary Constituency Sl.No Polling Location and name of building in Polling Areas Whether for All station No. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2013 [Price: Rs. 31.20 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 50] CHENNAI, WEDNESDAY, DECEMBER 25, 2013 Margazhi 10, Vijaya, Thiruvalluvar Aandu–2044 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 3585-3658 Notice ... 3659-3661 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 53999. My son, C. Vijay, son of Thiru G. Chandrasekaran, 54002. I, P. Pandiselvi, wife of Thiru M. Pandian, born on 25th November 1999 (native district: Virudhunagar), born on 26th June 1982 (native district: Madurai), residing at No. 258A, Nethaji Nagar, Kalappakulam, residing at No. 5-5-5/2, Valayakara Street, Sholavandan, Sankarankoil Taluk, Tirunelveli-627 756, shall henceforth be Vadipatti, Madurai-625 214, shall henceforth be known as C. VIJAYAKUMAR. known as P PANDEESWARI. C. SHENBAGADEVI. ð£. 𣇮ªê™M. Tirunelveli, 16th December 2013. (Mother.) Madurai, 16th December 2013. 54000. My daughter, R. Evangeline, born on 24th July 54003. I, A. Shanmugaraja, son of Thiru M. Ayyanan, 1996 (native district: Virudhunagar), residing at No. 4/2A, born on 18th May 1986 (native district: Madurai), residing at Mettukundu, Indira Colony, Virudhunagar-645 618, shall No. 63, Pasupathi Nagar, P&T Nagar, Madurai-625 017, henceforth be known as A. -
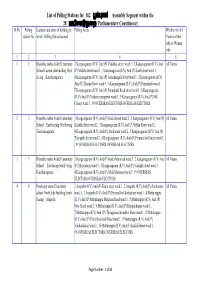
List of Polling Stations for 162 »Ù« Uü Assembly Segment Within the 28
List of Polling Stations for 162 _uü Assembly Segment within the 28 Parliamentary Constituency jkிnuº |m Sl.No Polling Location and name of building in Polling Areas Whether for All station No. which Polling Station located Voters or Men only or Women only 1 2 3 4 5 1 1 Bharatha matha Aided Elementary 1.Kanjanagaram (R.V) And (P) Vadakku street ward:1 , 2.Kanjanagaram (R.V) And All Voters School Eastern side building West (P) Middle Street ward:1 , 3.Kanjanagaram (R.V) And (P) South street ward:1 , facing ,Kanchanagaram 4.Kanjanagaram (R.V) And (P) Asdamangala Street ward:3 , 5.Kanjanagaram (R.V) And (P) Thoppu Street ward:3 , 6.Kanjanagaram (R.V) And (P) Ponnukud iward:1 , 7.Kanjanagaram (R.V) And (P) Ponnukudi Road street ward:1 , 8.Kanjanagaram (R.V) And (P) Nathavarayanpettai ward:1 , 9.Kanjanagaram (R.V) And (P) Mill Colony ward:3 , 99.OVERSEAS ELECTORS OVERSEAS ELECTORS 2 2 Bharatha matha Aided Elementary 1.Kanganagaram (R.V) And (P) Keela Street ward:2 , 2.Kanganagaram (R.V) And (P) All Voters School ,East faceing North wing Kulathu Street ward:2 , 3.Kanganagaram (R.V) And (P) Vellan Street ward:2 , ,Kanchanagaram 4.Kanganagaram (R.V) And (P) Akraharam ward:2 , 5.Kanganagaram (R.V) And (P) Thenpathi Street ward:2 , 6.Kanganagaram (R.V) And (P) Perumal koil Street ward:2 , 99.OVERSEAS ELECTORS OVERSEAS ELECTORS 3 3 Bharatha matha Aided Elementary 1.Kanganagaram (R.V) And (P) Keela Mainroad ward:3 , 2.Kanganagaram (R.V) And All Voters School , East facing South wing (P) Melacolony ward:3 , 3.Kanganagaram (R.V) And (P) Vadakku street -

Mayiladuthurai
MAYILADUTHURAI NAME OF S. NO ROLL.NO ADDRESS ADVOCATE NO.55, SARATHATTAI ST, RAILADY, MAYILADUTHURAI-3. 1 2/1979 ALAGANANDAM K. 1/113, VETRI VINAYAGAR NAGAR, MAPPADUGAI, MAYILADUTHURAI - 609 003 2 1137/2014 ALAGIRI N. S. NO.29, MATHARASA ST, ELANDANGUDI-609401, ARIVALUR-VLG, KUTTALAM-TK, NAGAI-DT. 3 201/1981 AMANULLAH S.A. NO.61, A.T.PURAM ROAD, SENTHANGUDI, MAYILADUTHURAI 609001 4 2174/2005 AMIRTHARAJAN D. 20/11, ALWARKULAM, THIRUVILANDUR MAYILADUTHURAI TALUK, NAGAPATTINAM 5 697/1996 ANBUROSE P. 26/5, WEST STREET ARCADU, ANANTHANDAVAPURAM POST, MAYILADUTHURAI TALUK, NAGAPATTINAM. 6 1920/2007 ARIVOLI P. MAIN ROAD, VANATHIRAJA PURAM POST, KUTHALAM TALUK, NAGAI DIST. 7 1194/1997 ARMSTRONG M. SOUTH STREET, AUTHUKUDI POST, MAYILADUTHURAI TALUK, NAGAPATTINAM DT 8 869/2014 ARUL D. NO.4/199, PALAVELI MAIN ROAD, VILANAGER, ARUPATHY-P, TRANQUEBAR-TK, NAGAI- DT.609309. 9 926/1990 ARUL NAMBI C. PUTHAGARAM, THIRUVALAPPUTHUR (POST), MAYILADUTHURAI (T,K) NAGAI DISTRICT, PIN - 609205. 10 961/1999 ARULDOSS Y. NAME OF S. NO ROLL.NO ADDRESS ADVOCATE WEST STREET, MANAKUDI POST, MAYILADUTHURAI TALUK, NAGAPATTINAM DIST - 609 118. 11 1044/2012 ARULTHAS C. THENPATHY STREET, KATHIRUPPU POST, SIRKALI TALUK, NAGAI DIST - 609 109. 12 2648/2012 ARUNA A. NO.172, PANANTHOPPU ST, SITHAARKADU, MAYILADUTHURAI, NAGAPPATTINAM-DT- 609003. 13 269/2002 BALAJI N. MAIN ROAD, MAPPADUGAI POST, MAYURAM TALUK, MAYILADUTHURAI,NAGAPATTINAM DT 14 569/1977 BALAKRISHNAN M. 42, CHINNAKANNARA ST., MAYILADUTHURAI TOWN, NAGAPATTINAM DT. 609001. 15 1856/2008 BALAMURUGAN K. NO.10. SIVAN EAST STREET, THERIZHANDUR (POST), KUTTHALAM TALUK, NAGAI, PIN - 609808. 16 1085/2008 BALAMURUGAN K. PLOT NO.5, P.M.G. NAGAR, MAYILADUTHURAI, BALASUBRAMANIA NAGAPPATTINAM DISTRICT. -
Private Schools Fee Determination Committee Chennai-600 006 - Fees Fixed for the Year 2013-2016 - District: Nagapattinam Sl
PRIVATE SCHOOLS FEE DETERMINATION COMMITTEE CHENNAI-600 006 - FEES FIXED FOR THE YEAR 2013-2016 - DISTRICT: NAGAPATTINAM SL. SCHOOL HEARING SCHOOL NAME & ADDRESS YEAR LKG UKG I II III IV V VI VII VIII IX X XI XII NO. CODE DATE 2013 - 14 3500 3500 4000 4000 4000 4500 4500 - - - - - - - Al-Islaah Nursery & Primary School 1 210001 4, Nool Kadai Street, 16-05-2013 2014 - 15 3850 3850 4400 4400 4400 4950 4950 - - - - - - - Nagore, Nagapattinam - 611 002. 2015 - 16 4235 4235 4840 4840 4840 5445 5445 - - - - - - - Golden Public Nursery & 2013 - 14 4400 4400 5860 5860 5860 5860 5860 - - - - - - - Primary School No. 15/OA 2 210003 14-03-13 2014 - 15 4840 4840 6446 6446 6446 6446 6446 - - - - - - - Cutchery Road Mayiladuthurai Nagapattinam Dist 2015 - 16 5324 5324 7091 7091 7091 7091 7091 - - - - - - - Jayam Nursery & Primary 2013 - 14 3800 3800 4220 4220 4220 4220 4220 - - - - - - - School 4/273, Main Road, 3 210005 16-05-2013 2014 - 15 4180 4180 4642 4642 4642 4642 4642 - - - - - - - Thirumullaivasal, Sirkali Taluk, Nagai - 609 113. 2015 - 16 4598 4598 5107 5107 5107 5107 5107 - - - - - - - 2013 - 14 5000 5000 6100 6100 6100 6100 6100 - - - - - - - M.R.T. Vidyalaya North Street, 4 210007 16-05-2013 2014 - 15 5500 5500 6710 6710 6710 6710 6710 - - - - - - - Kilvelur -611 104, Nagapattinam Dist. 2015 - 16 6050 6050 7381 7381 7381 7381 7381 - - - - - - - Mount Carmel Convent 2013 - 14 4500 4500 5500 5500 5500 6000 6000 - - - - - - - Nursery & Primary School 5 210009 Main Road, 16-05-2013 2014 - 15 4950 4950 6050 6050 6050 6600 6600 - - - - - - - Erukkur Post, Sirkali, Nagapattinam - 609 109. 2015 - 16 5445 5445 6655 6655 6655 7260 7260 - - - - - - - 2013 - 14 2540 2540 - - - - - - - - - - - - NAGA NURSERY & PRIMARY SCHOOL 6 210010 KAMESWAWARAN WEST 14-3-13 2014 - 15 2794 2794 - - - - - - - - - - - - KEEVALUR NAGAPATTINAM 2015 - 16 3074 3074 - - - - - - - - - - - - 1 PRIVATE SCHOOLS FEE DETERMINATION COMMITTEE CHENNAI-600 006 - FEES FIXED FOR THE YEAR 2013-2016 - DISTRICT: NAGAPATTINAM SL. -

Aqar 2018-2019
Yearly Status Report - 2018-2019 Part A Data of the Institution 1. Name of the Institution A.V.C. COLLEGE (AUTONOMOUS) Name of the head of the Institution Dr. R. NAGARAJAN Designation Principal Does the Institution function from own campus Yes Phone no/Alternate Phone no. 04364222264 Mobile no. 9487112627 Registered Email [email protected] Alternate Email [email protected] Address Mannampandal, Mayiladuthurai, Nagapattinam District, Tamil Nadu City/Town Mayiladuthurai State/UT Tamil Nadu Pincode 609305 2. Institutional Status Autonomous Status (Provide date of Conformant of 24-Jun-1987 Autonomous Status) Type of Institution Co-education Location Rural Financial Status state Name of the IQAC co-ordinator/Director Dr. A Govindarasu Phone no/Alternate Phone no. 04364229225 Mobile no. 9443715848 Registered Email [email protected] Alternate Email [email protected] 3. Website Address Web-link of the AQAR: (Previous Academic Year) http://www.avccollege.net/pdf/aqar2017- 18.pdf 4. Whether Academic Calendar prepared during Yes the year if yes,whether it is uploaded in the institutional website: Weblink : http://www.avccollege.net/pdf/Calendar_ 2018_2019.pdf 5. Accrediation Details Cycle Grade CGPA Year of Validity Accrediation Period From Period To 3 A 3.34 2013 23-Mar-2013 22-Mar-2018 2 A 0 2006 17-Oct-2006 16-Oct-2011 1 Four Star 0 2000 07-Feb-2000 06-Feb-2005 6. Date of Establishment of IQAC 29-Jun-2004 7. Internal Quality Assurance System Quality initiatives by IQAC during the year for promoting quality culture Item /Title -
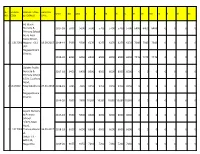
Nagapattinam District
SL. SCHOOL SCHOOL NAME HEARING YEAR LKG UKG I II III IV V VI VII VIII IX X XI XII NO. CODE & ADDRESS DATE AL-Islaah Nursery & 2017-18 5000 5000 5700 5700 5700 5700 5700 6400 6400 6400 0 0 0 0 Primary School No.4, Nool Kadai Street, 1 1317001 Nagore - 611 19.09.2017 2018-19 5500 5500 6270 6270 6270 6270 6270 7040 7040 7040 0 0 0 0 002. Nagapattinam District. 2019-20 6050 6050 6900 6900 6900 6900 6900 7745 7745 7745 0 0 0 0 Golden Public Nursery & 2017-18 6450 6450 8500 8500 8500 8500 8500 0 0 0 0 0 0 0 Primary School 15/A, Cutchery Road, 2 1317002 Mayiladuthura 24.01.2018 2018-19 7095 7095 9350 9350 9350 9350 9350 0 0 0 0 0 0 0 i, Nagapattinam District. 2019-20 7805 7805 10285 10285 10285 10285 10285 0 0 0 0 0 0 0 Jayam Nursery & Primary 2017-18 5500 5500 6000 6000 6000 6000 6000 0 0 0 0 0 0 0 School 4/273, Main Road, 3 1317003 Thirumullaivas 26.09.2017 2018-19 6050 6050 6600 6600 6600 6600 6600 0 0 0 0 0 0 0 al, Sirkali T.K. - 609 113, Nagai Dist. 2019-20 6655 6655 7260 7260 7260 7260 7260 0 0 0 0 0 0 0 SL. SCHOOL SCHOOL NAME HEARING YEAR LKG UKG I II III IV V VI VII VIII IX X XI XII NO. CODE & ADDRESS DATE M.R.T.Vidhyala ya Nursery & 2017-18 7000 7000 8000 8000 8000 8000 8000 0 0 0 0 0 0 0 Primary School, North Street, 4 1317004 Kivelur - 611 08.06.2017 2018-19 7700 7700 8800 8800 8800 8800 8800 0 0 0 0 0 0 0 104 Nagapattinam Dist. -

Nagapattinam-Paddy II-STP19-20-Villagewise Claim Details
Agriculture Insurance Company of India Ltd, Regional Office, Chennai PMFBY-STP19-20-Rabi season-Claim details-Nagapattinam district Area Insured Claim Amount Block Crop Village Name (ha) (Rs.) Keelaiyur Karappidagai (North) 196.44 0 Keelaiyur Karappidagai (South) 176.13 0 Keelaiyur Keelaiyur 487.49 694139 Keelaiyur Kilapidagai 369.69 0 Keelaiyur Prathabaramapuram 486.00 0 Keelaiyur Thiruppoondi (East) 310.20 0 Keelaiyur Thiruppoondi (West) 364.89 0 Keelaiyur Venmanacheri 575.30 0 Keelaiyur Vettaikkaraniruppu 122.86 0 Keelaiyur Vizhunthamavadi 225.41 0 Keelaiyur Esanur 182.65 0 Keelaiyur Ettugudi 319.22 0 Keelaiyur Madapuram 298.72 0 Keelaiyur Malavelakarai 373.82 8688613 Keelaiyur Meenamanallur 344.33 0 Keelaiyur Thirukuvalai 483.72 2213341 Keelaiyur Thiruvaimur 437.45 0 Keelaiyur Valakarai 300.10 0 Keelaiyur Vallam 160.57 0 Keelaiyur Chinathumboor 408.04 0 Keelaiyur Cholavidyapuram 355.31 3962631 Keelaiyur Erayankudy 280.96 0 Keelaiyur Karunguni 411.78 0 Keelaiyur Ottathattai 176.64 0 Keelaiyur Palakurichi 391.32 0 Keelaiyur Thalayamazhai 248.00 0 Keelaiyur Thanilappadi 251.10 0 Keelaiyur Vappanchery 199.88 0 Keelaiyur Velankanni 20.85 0 Kilvelur Agarakadambanur 365.54 0 Kilvelur Anaimangalam 405.23 5215207 Kilvelur Athipuliyur 277.06 10745435 Kilvelur Eravancheri 266.69 0 Kilvelur Iluppur 175.35 0 Kilvelur Kilvelur 330.55 6250178 Kilvelur Kohur 289.93 0 Kilvelur Koothur 193.13 4522489 Kilvelur Kurukkathi 92.70 0 Kilvelur Kurumanangudi 231.37 7390924 Kilvelur Okkur 439.83 12323963 Kilvelur Pattamangalam 324.99 0 Kilvelur Serunallur 167.02