Drug Trend Bulletin – Issue 13 September 2016
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Benzodiazepine Group ELISA Kit
Benzodiazepine Group ELISA Kit Benzodiazepine Background Since their introduction in the 1960s, benzodiazepines have been widely prescribed for the treatment of anxiety, insomnia, muscle spasms, alcohol withdrawal, and seizure-prevention as they are depressants of the central nervous system. Despite the fact that they are highly effective for their intended use, benzodiazepines are prescribed with caution as they can be highly addictive. In fact, researchers at NIDA (National Institute on Drug Abuse) have shown that addiction for benzodiazepines is similar to that of opioids, cannabinoids, and GHB. Common street names of benzodiazepines include “Benzos” and “Downers”. The five most encountered benzodiazepines on the illicit market are alprazolam (Xanax), lorazepam (Ativan), clonazepam (Klonopin), diazepam (Valium), and temazepam (Restori). The method of abuse is typically oral or snorted in crushed form. The DEA notes a particularly high rate of abuse among heroin and cocaine abusers. Designer benzodiazepines are currently offered in online shops selling “research chemicals”, providing drug abusers an alternative to prescription-only benzodiazepines. Data defining pharmacokinetic parameters, drug metabolisms, and detectability in biological fluids is limited. This lack of information presents a challenge to forensic laboratories. Changes in national narcotics laws in many countries led to the control of (phenazepam and etizolam), which were marketed by pharmaceutical companies in some countries. With the control of phenazepam and etizolam, clandestine laboratories have begun researching and manufacturing alternative benzodiazepines as legal substitutes. Delorazepam, diclazepam, pyrazolam, and flubromazepam have emerged as compounds in this class of drugs. References Drug Enforcement Administration, Office of Diversion Control. “Benzodiazepines.” http://www.deadiversion.usdoj.gov/drugs_concern/benzo_1. -

The Misuse of Benzodiazepines Among High-Risk Opioid Users in Europe
EMBARGO — 7 JUNE 7. 6. 2018 UPDATED 11:30 Central European Time/CET (10:30 Western European Time/WET/Lisbon) Proof - 28 May 2018 not for circulation PERSPECTIVES ON DRUGS The misuse of benzodiazepines among high-risk opioid users in Europe Benzodiazepines are a widely prescribed I Introduction group of medicines with a range of clinical uses that include treating Benzodiazepines have a range of clinical uses and are among the most commonly prescribed medicines globally. anxiety, insomnia and managing alcohol They are useful in the short-term treatment of anxiety and withdrawal. This group of medicines is insomnia, and in managing alcohol withdrawal (Medicines often misused by high-risk opioid users, and Healthcare Products Regulatory Agency, 2015). Like all medicines, benzodiazepines can produce side effects. They and this is associated with considerable may also be misused, which we define as use without a morbidity and mortality. This paper prescription from a medical practitioner or, if prescribed, when describes the impact of benzodiazepines they are used outside accepted medical practice or guidelines. misuse on the health and treatment of While the misuse of benzodiazepines has been identified high-risk opioid users. as a concern for large groups in the general population, for example, among elderly people and women, this analysis focuses specifically on misuse among high-risk opioid users (1), a group of people among whom these medicines have been linked with severe treatment challenges and implicated in considerable numbers of drug-related deaths. It is important to stress that much benzodiazepine prescribing to high-risk drug users is done with legitimate therapeutic aims in mind. -

Flualprazolam Article Originally Appeared in TOXTALK®, Volume 43, Issue 4
Donna Papsun¹, MS, D-ABFT-FT, Craig Triebold², F-ABC, D-ABFT-FT Emerging Drug: ¹NMS Labs, Horsham, PA; ²Sacramento County District Attorney Laboratory of Forensic Services, Sacramento, CA Flualprazolam Article originally appeared in TOXTALK®, Volume 43, Issue 4 In recent years, there has been an increase of misuse related to designer benzodiazepines (DBZD), a subcategory of novel psychoactive substances (NPS). Benzodiazepines are commonly prescribed for their anxiolytic, muscle relaxant, sedative- hypnotic, and anticonvulsant properties, but due to their widespread availability and relatively low acute toxicity, there is a high potential for misuse and dependence. Therefore, in the era of analogs of commonly used substances emerging on the drug market as suitable alternatives, it is not unexpected that designer variants of benzodiazepines have become available and in demand. Compounds of this class may have either been repurposed from pharmaceutical research, chemically modified from prescribed benzodiazepines, or obtained from diversion of pharmaceuticals available in other countries. Flualprazolam, a fluorinated analog of alprazolam, is an emerging designer benzodiazepine with increasing prevalence, which is an example of a modification to a prescribed benzodiazepine. It was first patented in the 1970s but never marketed, so it has been repurposed for recreational abuse from pharmaceutical research as well (1). Its chemical characteristics and structure are listed in Figure 1. Flualprazolam is a high potency triazolo-benzodiazepine with sedative effects similar to other benzodiazepines (2). It is marketed by internet companies for “research purposes” as an alternative to alprazolam and discussions on online forums suggest that flualprazolam lasts longer and is stronger than alprazolam, its non-fluorinated counterpart (3). -

The Emergence of New Psychoactive Substance (NPS) Benzodiazepines
Issue: Ir Med J; Vol 112; No. 7; P970 The Emergence of New Psychoactive Substance (NPS) Benzodiazepines. A Survey of their Prevalence in Opioid Substitution Patients using LC-MS S. Mc Namara, S. Stokes, J. Nolan HSE National Drug Treatment Centre Abstract Benzodiazepines have a wide range of clinical uses being among the most commonly prescribed medicines globally. The EU Early Warning System on new psychoactive substances (NPS) has over recent years detected new illicit benzodiazepines in Europe’s drug market1. Additional reference standards were obtained and a multi-residue LC- MS method was developed to test for 31 benzodiazepines or metabolites in urine including some new benzodiazepines which have been classified as New Psychoactive Substances (NPS) which comprise a range of substances, including synthetic cannabinoids, opioids, cathinones and benzodiazepines not covered by international drug controls. 200 urine samples from patients attending the HSE National Drug Treatment Centre (NDTC) who are monitored on a regular basis for drug and alcohol use and which tested positive for benzodiazepine class drugs by immunoassay screening were subjected to confirmatory analysis to determine what Benzodiazepine drugs were present and to see if etizolam or other new benzodiazepines are being used in the addiction population currently. Benzodiazepine prescription and use is common in the addiction population. Of significance we found evidence of consumption of an illicit new psychoactive benzodiazepine, Etizolam. Introduction Benzodiazepines are useful in the short-term treatment of anxiety and insomnia, and in managing alcohol withdrawal. 1 According to the EMCDDA report on the misuse of benzodiazepines among high-risk opioid users in Europe1, benzodiazepines, especially when injected, can prolong the intensity and duration of opioid effects. -

NMS Labs Demo Report
NMS Labs CONFIDENTIAL 200 Welsh Road, Horsham, PA 19044-2208 Phone: (215) 657-4900 Fax: (215) 657-2972 e-mail: [email protected] Robert A. Middleberg, PhD, F-ABFT, DABCC-TC, Laboratory Director Demo Report Patient Name 0570B-POS Report Issued 06/19/2017 08:16 Patient ID 0570B-POS Last Report Issued 04/10/2017 13:19 Chain 17000768 Age Not Given DOB Not Given 88888 Gender Not Given Clinical Example Report Attn: IT Department Workorder 17000768 200 Welsh Road Horsham, PA 19044-2208 Received 04/10/2017 12:42 Sample ID 17000768-001 Collect Dt/Tm Not Given Matrix Blood Source Not Given Patient Name 0570B-POS Patient ID 0570B-POS Container Type Clear vial Approx Vol/Weight Not Given Receipt Notes None Entered Reporting Analysis and Comments Result Units Limit Notes 0570B Designer Benzodiazepines, Blood (Forensic) Analysis by High Performance Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS) Bromazepam 50 ng/mL 5.0 Bromazepam is a benzodiazepine drug that is used as a novel psychoactive substance. It is reported to have CNS depressant properties and shares anticonvulsant, muscle relaxant, hypnotic, anxiolytic and sedative effects with other benzodiazepines. It is not approved for use in the United States, but is available in some other countries. Average peak plasma concentrations following a single 3 mg, 6 mg and 12 mg dose were reported to be 10 ng/mL at 8 hours, 83 ng/mL at 2 hours and 130 ng/mL at 1-4 hours after dosing, respectively. Chronic oral administration of 9 mg daily resulted in an steady-state plasma concentrations of 81-150 ng/mL (Average = 120 ng/mL). -

A Review of the Evidence of Use and Harms of Novel Benzodiazepines
ACMD Advisory Council on the Misuse of Drugs Novel Benzodiazepines A review of the evidence of use and harms of Novel Benzodiazepines April 2020 1 Contents 1. Introduction ................................................................................................................................. 4 2. Legal control of benzodiazepines .......................................................................................... 4 3. Benzodiazepine chemistry and pharmacology .................................................................. 6 4. Benzodiazepine misuse............................................................................................................ 7 Benzodiazepine use with opioids ................................................................................................... 9 Social harms of benzodiazepine use .......................................................................................... 10 Suicide ............................................................................................................................................. 11 5. Prevalence and harm summaries of Novel Benzodiazepines ...................................... 11 1. Flualprazolam ......................................................................................................................... 11 2. Norfludiazepam ....................................................................................................................... 13 3. Flunitrazolam .......................................................................................................................... -

Toxicology Drug Testing Panels
RTXMSJ06008UAH Toxicology Drug Testing Panels Document Number: RTXMSJ06008UAH Revision Number: 1.73 Document Type: Attachment Effective Date: 5/3/2021 3:08:59 PM Location: AHS Laboratory Services\Document Administration\Toxicology and Trace Elements\17. Drug Testing LCMSMS\1. Drug Testing LCMSMS Job Aids RTXMSJ06008UAH Toxicology Drug Testing Panels Urine Opioid Dependency Panel 1 (UODP) CUT-OFF DRUG CLASS COMMON TRADE NAMES INTERPRETIVE CONCENTRATION2 ANALYTE DETECTED (STREET NAME) NOTES (NG/ML) Amphetamines Adderall, Dexedrine, Vyvanse, A prescription drug; also a metabolite of Amphetamine 250 Lisdexamfetamine methamphetamine (Speed, Bennies, Crystal Meth, Metabolite of Selegiline; Metabolized to Methamphetamine 250 Uppers) amphetamine Metabolite of Methylenedioxyamphetamine (MDA) 250 methylenedioxymethamphetamine Methylenedioxymethamphetamine (Ecstasy, MDMA) Metabolized to methylenedioxyamphetamine 250 Page 1 of 15 RTXMSJ06008UAH Toxicology Drug Testing Panels Rev: 1.73 CUT-OFF DRUG CLASS COMMON TRADE NAMES INTERPRETIVE CONCENTRATION2 ANALYTE DETECTED (STREET NAME) NOTES (NG/ML) Benzodiazepines Flubromazepam prescription not available in Flubromazepam 50 Canada 7-Aminoclonazepam Metabolite of clonazepam (Rivotril) 50 7-Aminonitrazepam Metabolite of nitrazepam (Mogadon) 50 Alphahydroxyalprazolam Metabolite of alprazolam (Xanax) 50 Alphahydroxytriazolam Metabolite of triazolam (Halcion) 50 Bromazepam Lectopam 50 Clobazam Frisium Metabolized to norclobazam 50 o Norclobazam Metabolite of clobazam 50 Metabolite of chlordiazepoxide -
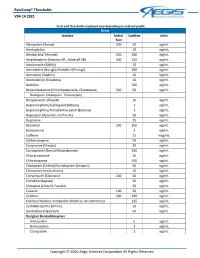
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam -

Forensic Toxicology Scope of Testing and Detection Limits
J. RYAN MCMAHON II County Executive INDU GUPTA, MD, MPH MEDICAL EXAMINER’S OFFICE Commissioner of Health FORENSIC TOXICOLOGY LABORATORY CAROLYN H. REVERCOMB, MD, DABP ONONDAGA COUNTY HEALTH DEPARTMENT Chief Medical Examiner KRISTIE BARBA, MS, D-ABFT-FT CENTER FOR FORENSIC SCIENCES Toxicologist Forensic Toxicology Scope of Testing and Detection Limits Table of Contents QUALITATIVE ANALYSES.........................................................................................................2 Volatile Screen by GC/FID .............................................................................................................2 Carbon Monoxide by Microdiffusion..............................................................................................2 Carbon Monoxide by CO-Oximeter ................................................................................................2 Ethylene Glycol by GC/MS.............................................................................................................2 ELISA Buprenorphine by IA for Blood/Urine ................................................................................2 ELISA by IA for Blood ...................................................................................................................3 ELISA by IA for Urine....................................................................................................................4 Gamma Hydroxybutyrate (GHB) by GC/MS..................................................................................5 Gabapentin, -

Advisory Council on the Misuse of Drugs
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen NPS Committee Secretary: Linsey Urquhart 1st Floor (NE), Peel Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 1121 [email protected] Sarah Newton MP Minister for Vulnerability, Safeguarding and Countering Extremism Home Office 2 Marsham Street London SW1P 4DF 2 December 2016 Dear Minister, I am writing to recommend that you lay a temporary class drug order (TCDO) pursuant to section 2A of the Misuse of Drugs Act 1971 (MDA) for the following substances: • U-47,700 • Etizolam and other designer benzodiazepines. Please find enclosed two reports containing the Advisory Council on the Misuse of Drugs’ (ACMD) consideration of the evidence of harms on these substances. U-47,700 U-47,700 is a synthetic opioid, originally developed as a research chemical but with no legitimate use. Reportedly 7.5 times more potent than morphine it is a structural analogue of AH-7921. AH-7921 was controlled as a Class A drug in January 2015 following ACMD advice, particularly regarding its high addiction potential. The ACMD is concerned that abuse of U-47,700 has the potential for severe harms, particularly following reports from the USA of more than 80 deaths attributed to this substance and that the patterns of abuse are mirroring those of heroin. The US Drug Enforcement Administration has consequently subjected U-47,700 to temporary emergency scheduling under the Controlled Substances Act. Designer Benzodiazepines Benzodiazepines such as diazepam and chlordiazepoxide have had medical applications for more than 50 years, particularly as sedatives. -

FLUBROMAZOLAM (Street Name: Liquid Xanax)
Drug Enforcement Administration Diversion Control Division Drug & Chemical Evaluation Section FLUBROMAZOLAM (Street Name: Liquid Xanax) August 2019 DEA/DC/DO/DOE Introduction (0.5 mg/day) of flubromazolam, sedative-hypnotic effects as Flubromazolam is a triazole analogue of the designer well as muscle relaxant effects occurred 90 minutes following benzodiazepine, flubromazepam. As a class of drugs, drug intake. Drowsiness occurred approximately three hours benzodiazepines produce central nervous system (CNS) post-drug ingestion, and lasted for five hours. Intoxication due depression and are commonly used to treat, panic disorders, to flubromazolam is characterized by excessive drowsiness, anxiety and insomnia. The United States Food and Drug partial amnesia and inability to follow or participate in Administration has not approved Flubromazolam for conversation. Peak serum concentration of flubromazolam is therapeutic use. reached approximately 5 hours (7.4 ng/mL) after administration with a second peak occurring after 8 hours (8.6 ng/mL), making Licit Uses it a long-acting benzodiazepine. In a single-dose Flubromazolam does not currently have an accepted pharmacokinetic study in humans, 30 hours following medical use in the United States. flubromazolam ingestion, a re-emergence of sedative effects was observed. Chemistry Flubromazolam (8-bromo-6-(2-fluorophenyl)-1-methyl- User Population 4H-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepine) is a triazole Flubromazolam is used as a recreational substance in the United States. It is abused by a broad range of groups analogue of the benzodiazepine flubromazepam. including youths, young adults, and older adults. Flubromazolam is composed of a benzene ring fused to a seven-membered 1,4-diazepine ring that is also fused to a 1,2,4 Illicit Distribution triazole ring. -

Applications
TN-0143 APPLICATIONS Designer and Pharmaceutical Benzodiazepine Analysis from Urine using In-Well Hydrolysis Solid Phase Extraction (SPE) and LC-MS/MS Brittany LeBlanc Phenomenex, Inc., 411 Madrid Ave., Torrance, CA 90501 USA Overview Sample Preparation Designer benzodiazepines are a rapidly increasing threat as an alternative to prescription benzodiazepines and due to their com- Pre-treatment plex structures and sample matrices, clean-up techniques can Combine 100 μL spiked urine, 30 μL IMCS Room Temp buffer, be difficult to develop. Designer benzos provide many analytical 270 µL water, and 10 μL of IMCSzyme RT onto the plate. Incu- challenges due to the limited knowledge of their metabolites and bate for 15 minutes at room temperature. the similarities to traditional pharmaceutical benzodiazepines, such as Diclazepam and its metabolite, Lorazepam. This can Solid Phase Extraction Method make clean-up and separation a problematic step in the method 96-Well Plate: Strata-X Drug B Plus, 30 mg/well development process. Part No.: 8E-S128-TGB-P Condition: Not required Equilibration: Not required In traditional sample preparation methods, the hydrolysis step Load: After incubation time is up, switch vacuum manifold on quickly to facilitate must be performed prior to the extraction step and analytes must flow through the SPE sorbent be converted back to their non-conjugated form, which often re- Wash 1: 1 mL of 1.0 % Formic acid in Water Wash 2: 1 mL of Water/Methanol (70:30) quires long wait time and high temperatures by either ovens or Dry: 10 minutes at high vacuum water baths. Once the hydrolysis has been performed, samples Elute: 2x 0.5 mL 5 % Ammonium hydroxide in Methanol/Acetonitrile (50:50) must be transferred to an extraction product which could cause Dry Down: To dryness at 40 °C under a gentle stream of Nitrogen Reconstitute: 100 µL 0.1% Formic acid in Water/0.1% Formic acid in Methanol (85:15) cross-contamination or loss of sample in the transfer step.