Author Version:Estuarine, Coastal and Shelf Science,Vol.196; 2017;10-21
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
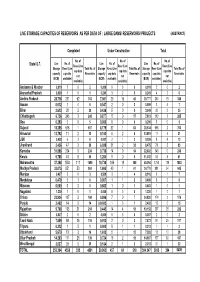
Live Storage Capacities of Reservoirs As Per Data of : Large Dams/ Reservoirs/ Projects (Abstract)
LIVE STORAGE CAPACITIES OF RESERVOIRS AS PER DATA OF : LARGE DAMS/ RESERVOIRS/ PROJECTS (ABSTRACT) Completed Under Construction Total No. of No. of No. of Live No. of Live No. of Live No. of State/ U.T. Resv (Live Resv (Live Resv (Live Storage Resv (Live Total No. of Storage Resv (Live Total No. of Storage Resv (Live Total No. of cap data cap data cap data capacity cap data Reservoirs capacity cap data Reservoirs capacity cap data Reservoirs not not not (BCM) available) (BCM) available) (BCM) available) available) available) available) Andaman & Nicobar 0.019 20 2 0.000 00 0 0.019 20 2 Arunachal Pradesh 0.000 10 1 0.241 32 5 0.241 42 6 Andhra Pradesh 28.716 251 62 313 7.061 29 16 45 35.777 280 78 358 Assam 0.012 14 5 0.547 20 2 0.559 34 7 Bihar 2.613 28 2 30 0.436 50 5 3.049 33 2 35 Chhattisgarh 6.736 245 3 248 0.877 17 0 17 7.613 262 3 265 Goa 0.290 50 5 0.000 00 0 0.290 50 5 Gujarat 18.355 616 1 617 8.179 82 1 83 26.534 698 2 700 Himachal 13.792 11 2 13 0.100 62 8 13.891 17 4 21 J&K 0.028 63 9 0.001 21 3 0.029 84 12 Jharkhand 2.436 47 3 50 6.039 31 2 33 8.475 78 5 83 Karnatka 31.896 234 0 234 0.736 14 0 14 32.632 248 0 248 Kerala 9.768 48 8 56 1.264 50 5 11.032 53 8 61 Maharashtra 37.358 1584 111 1695 10.736 169 19 188 48.094 1753 130 1883 Madhya Pradesh 33.075 851 53 904 1.695 40 1 41 34.770 891 54 945 Manipur 0.407 30 3 8.509 31 4 8.916 61 7 Meghalaya 0.479 51 6 0.007 11 2 0.486 62 8 Mizoram 0.000 00 0 0.663 10 1 0.663 10 1 Nagaland 1.220 10 1 0.000 00 0 1.220 10 1 Orissa 23.934 167 2 169 0.896 70 7 24.830 174 2 176 Punjab 2.402 14 -

Ecological Status of Kali River Flood Plain
Annexure 6 Ecological Status of Kali River Flood Plain Sahyadri Conservation Series: 8 ENVIS Technical Report: 29, October 2008 Environmental Information System [ENVIS] Centre for Ecological Sciences, Indian Institute of Science, Bangalore - 560012, INDIA Web: http://ces.iisc.ernet.in/hpg/envis http://ces.iisc.ernet.in/energy/ http://ces.iisc.ernet.in/biodiversity Email: [email protected], [email protected] 102 Ecological Status of Kali River Flood Plain Sr. No. Title Page No. 1 Summary 3 2 Introduction 6 3 Study area 15 4 Methods 21 5 Result and Discussion 23 6 Conclusion 49 7 Acknowledgment 49 8 References 50 Tables Sr.No Name Pg No. 1 List of organisms found in Western Ghats with their endemism percentage 8 2 Acts and policies in India for protecting environment and wildlife 11 3 Land use details in the drainage basin of River Kali 16 4 Shrubs of Kali flood plain 24 5 Herbs of Kali flood plain 24 6 Trees of Kali flood plain 26 7 Climbers of Kali flood plain 28 8 Ferns of Kali flood plain 28 9 Rare and Threatened plants of Kali flood plain 28 10 The water quality values for each month during the study period in Naithihole 33 11 The water quality values for each month during the study period in Sakthihalla 34 12 Amphibian species list recorded from Kali River Catchment 36 13 Birds of Kali River Flood Plains 38 14 Water birds of the study area 40 Figures Sr.No Title Sr. No. Page No. 1 Study area – The flood plains of Kali River 17 2 Drainage network in Kali River basin 18 3 Mean Annual Rainfall in Kali River Basin 18 4 Land -

Dams-In-India-Cover.Pdf
List of Dams in India List of Dams in India ANDHRA PRADESH Nizam Sagar Dam Manjira Somasila Dam Pennar Srisailam Dam Krishna Singur Dam Manjira Ramagundam Dam Godavari Dummaguden Dam Godavari ARUNACHAL PRADESH Nagi Dam Nagi BIHAR Nagi Dam Nagi CHHATTISGARH Minimata (Hasdeo) Bango Dam Hasdeo GUJARAT Ukai Dam Tapti Dharoi Sabarmati river Kadana Mahi Dantiwada West Banas River HIMACHAL PRADESH Pandoh Beas Bhakra Nangal Sutlej Nathpa Jhakri Dam Sutlej Chamera Dam Ravi Pong Dam Beas https://www.bankexamstoday.com/ Page 1 List of Dams in India J & K Bagihar Dam Chenab Dumkhar Dam Indus Uri Dam Jhelam Pakal Dul Dam Marusudar JHARKHAND Maithon Dam Maithon Chandil Dam Subarnarekha River Konar Dam Konar Panchet Dam Damodar Tenughat Dam Damodar Tilaiya Dam Barakar River KARNATAKA Linganamakki Dam Sharavathi river Kadra Dam Kalinadi River Supa Dam Kalinadi Krishna Raja Sagara Dam Kaveri Harangi Dam Harangi Narayanpur Dam Krishna River Kodasalli Dam Kali River Basava Sagara Krishna River Tunga Bhadra Dam Tungabhadra River, Alamatti Dam Krishna River KERALA Malampuzha Dam Malampuzha River Peechi Dam Manali River Idukki Dam Periyar River Kundala Dam Parambikulam Dam Parambikulam River Walayar Dam Walayar River https://www.bankexamstoday.com/ Page 2 List of Dams in India Mullaperiyar Dam Periyar River Neyyar Dam Neyyar River MADHYA PRADESH Rajghat Dam Betwa River Barna Dam Barna River Bargi Dam Narmada River Bansagar Dam Sone River Gandhi Sagar Dam Chambal River . Indira Sagar Narmada River MAHARASHTRA Yeldari Dam Purna river Ujjani Dam Bhima River Mulshi -

Proposed Action Plan for Rejuvenation of River Kali
ACTION PLAN FOR REJUVENATION OF RIVER Kali ________________________________________________________________________________ 1 Proposed Action Plan for Rejuvenation of River Kali Karnataka State Pollution Control Board “Parisara Bhavana”, # 49, Church Street, Bengaluru - 560 001 January 2019 ACTION PLAN FOR REJUVENATION OF RIVER Kali ________________________________________________________________________________ 2 INDEX Topic Page No. Sl. No. 3-4 1 Introduction to Kali River 2 Sources of Pollution - Municipal Sewage 5 generation and Treatment 3 Characteristics of River water quality 6 4 Action taken by the Board 6 5 Action to be taken for Rejuvenation of River 6 Water Quality Cost component involved in the Restoration of 6 Polluted stretch 7 Status of Environmental Flow (E-Flow) 7 7-8 Short Term and Long Term Action and the 8 Identified Authorities for initiating actions and 8-12 the time limits for ensuring compliance ACTION PLAN FOR REJUVENATION OF RIVER Kali ________________________________________________________________________________ 3 Proposed action plan for Rejuvenation of River Kali 09. State : Karnataka River Name: Kali River Stretch : Hasan Maad (west coast paper mill) to Bommanahalli Reservoir Priority : IV (BOD 6-10 mg/L) BOD Max.value: 6.5 mg/L ___________________________________________________________________ 1.The Kali river rises near Diggi, a small village in Joida taluk, Uttar Kannada district. The Kali River is flowing in part of 5 taluks out of 11 taluks through Uttara Kannada district of Karnataka State. The river is the lifeline to some four lakh peoples in the Uttara Kannada district and supports the livelihoods of thousands of people including fishermen on the coast of Karwar. There are many dams built across this river for the generation of electricity. -
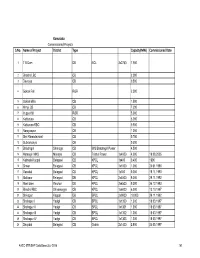
Karnataka Commissioned Projects S.No. Name of Project District Type Capacity(MW) Commissioned Date
Karnataka Commissioned Projects S.No. Name of Project District Type Capacity(MW) Commissioned Date 1 T B Dam DB NCL 3x2750 7.950 2 Bhadra LBC CB 2.000 3 Devraya CB 0.500 4 Gokak Fall ROR 2.500 5 Gokak Mills CB 1.500 6 Himpi CB CB 7.200 7 Iruppu fall ROR 5.000 8 Kattepura CB 5.000 9 Kattepura RBC CB 0.500 10 Narayanpur CB 1.200 11 Shri Ramadevaral CB 0.750 12 Subramanya CB 0.500 13 Bhadragiri Shimoga CB M/S Bhadragiri Power 4.500 14 Hemagiri MHS Mandya CB Trishul Power 1x4000 4.000 19.08.2005 15 Kalmala-Koppal Belagavi CB KPCL 1x400 0.400 1990 16 Sirwar Belagavi CB KPCL 1x1000 1.000 24.01.1990 17 Ganekal Belagavi CB KPCL 1x350 0.350 19.11.1993 18 Mallapur Belagavi DB KPCL 2x4500 9.000 29.11.1992 19 Mani dam Raichur DB KPCL 2x4500 9.000 24.12.1993 20 Bhadra RBC Shivamogga CB KPCL 1x6000 6.000 13.10.1997 21 Shivapur Koppal DB BPCL 2x9000 18.000 29.11.1992 22 Shahapur I Yadgir CB BPCL 1x1300 1.300 18.03.1997 23 Shahapur II Yadgir CB BPCL 1x1301 1.300 18.03.1997 24 Shahapur III Yadgir CB BPCL 1x1302 1.300 18.03.1997 25 Shahapur IV Yadgir CB BPCL 1x1303 1.300 18.03.1997 26 Dhupdal Belagavi CB Gokak 2x1400 2.800 04.05.1997 AHEC-IITR/SHP Data Base/July 2016 141 S.No. Name of Project District Type Capacity(MW) Commissioned Date 27 Anwari Shivamogga CB Dandeli Steel 2x750 1.500 04.05.1997 28 Chunchankatte Mysore ROR Graphite India 2x9000 18.000 13.10.1997 Karnataka State 29 Elaneer ROR Council for Science and 1x200 0.200 01.01.2005 Technology 30 Attihalla Mandya CB Yuken 1x350 0.350 03.07.1998 31 Shiva Mandya CB Cauvery 1x3000 3.000 10.09.1998 -
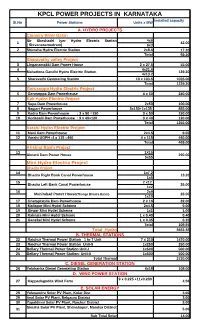
KPCL POWER PROJECTS in KARNATAKA Installed Capacity Sl.No Power Stations Units X MW
KPCL POWER PROJECTS IN KARNATAKA Installed capacity Sl.No Power Stations Units x MW A. HYDRO PROJECTS Cauvery River Basin Sir Sheshadri Iyer Hydro Electric Station 4x6 1 42.00 ( Shivanasamudram) 6x3 2 Shimsha Hydro Electric Station 2x8.6 17.20 Total 59.20 Sharavathy valley Project 3 Linganamakki Dam Power House 2 x 27.5 55.00 4 4x21.6 Mahathma Gandhi Hydro Electric Station 139.20 4x13.2 5 Sharavathi Generating Station 10 x 103.5 1035.00 Total 1229.20 Gerusoppa Hydro Electric Project 6 Gerusoppa Dam Powerhouse 4 x 60 240.00 Kali Hydro Electric Project 7 Supa Dam Powerhouse 2x50 100.00 8 Nagjari Powerhouse 5x150+1x135 885.00 9 Kadra Dam Powerhouse : 3 x 50 =150 3 x 50 150.00 10 Kodasalli Dam Powerhouse : 3 x 40=120 3 x 40 120.00 Total 1255.00 Varahi Hydro Electric Project 11 Mani Dam Powerhouse 2x4.5 9.00 12 Varahi UGPH :4 x 115 =460 4 x 115 460.00 Total 469.00 Krishna Basin Project 13 1X15 Almatti Dam Power House 290.00 5x55 Mini Hydro Electric Project Bhadra Project 14 1x7.2 Bhadra Right Bank Canal Powerhouse 13.20 1x6 15 2 x12 Bhadra Left Bank Canal Powerhouse 26.00 1x2 16 2x9 Munirabad Power House(Thunga Bhadra Basin) 28.00 1x10 17 Ghataprabha Dam Powerhouse 2 x 16 32.00 18 Mallapur Mini Hydel Scheme 2x4.5 9.00 19 Sirwar Mini Hydel Scheme 1x1 1.00 20 Kalmala Mini Hydel Scheme 1 x 0.40 0.40 21 Ganekal Mini Hydel Scheme 1 x 0.35 0.35 Total 109.95 Total Hydro 3652.35 B. -

River Otter Pilot Survey in the Kali River, Karwar, Uttara Kannada in Summer 2014
River Otter Pilot Survey in the Kali River, Karwar, Uttara Kannada in Summer 2014 Prepared by Rahul Kumar and Mridula Srinivasan Terra Marine Research Institute Project Summary Introduction and Background: Otters belong to the mammalian family Mustelidae (Subfamily Lutrinae). Otters are semi-aquatic fish eating mammals. India is home to three of the four species of river otters in Asia and of the thirteen species of otters found worldwide. The three commonly found river otters in India include common otter- Lutra lutra, smooth-coated otter- Lutrogale perspicillata, and small-clawed otter- Aonyx cinereus. The status of otters from the Indian subcontinent is poorly documented but anecdotal reports suggest that the population may be severely threatened from habitat destruction and poaching. River otters in India range throughout the country especially, smooth-coated otters. Small- clawed otters are sympatric in areas inhabited by smooth-coated otters. All 3 species are found in the Western Ghats and in northeast India. Protected areas have been established in northern, southern, and northeastern parts of India, but few have implemented habitat and conservation plans to understand population status and ecology and protect river otter populations. Breeding populations of otters have been recorded at Corbett and Dudhwa Tiger Reserve, Katerniaghat Wildlife Sanctuary, Kaziranga National Park, Sunderbans, Bhitarkanika and Coringa, Periyar Tiger Reserve and Nagarhole National Park. Otters in India are protected under Schedule II of Wildlife Protection Act of 1972 and are increasingly rare outside national parks and sanctuaries. It is likely that their populations outside of these protected reserves are at risk and poorly studied. Study Area: Karwar, Karnataka, located on the west coast of India is nestled between the Western Ghats mountain range to the east and the Arabian Sea to the west. -

Dossier of Overall Karnataka and 17 PRS in Compliance to the NGT Orders in the Matter of OA
Dossier of overall Karnataka and 17 PRS in compliance to the NGT orders in the matter of OA. 673/2018 State: Karnataka Estimated urban population: 22941508 1 Status of Sewage management: Overall Karnataka 17 PRS Estimated sewage generation 3356.5 MLD 884.25 MLD Existing no. of STPs and 141 STPs + 2 FSSM 40 STPs, Treatment Capacity Capacity = 2289.56 MLD Capacity = 635.54 MLD Capacity Utilization of 1592.4 MLD, 410 MLD, existing STPs percent utilization = 69.4% Percent utilization = 64.1% Sewage being treated through 4.53 MLD through FSSM (2.26 Insitu bioremediation: proposed for the Alternate technology (MLD) MLD in Devanahalli and 2.27 construction of artificial island in Dorekere MLD in Nelamangala) (global village Techpark) and Kempambudhi (Sonnenahalli bridge) amounting to ₹62.41 lakh Gap in Treatment Capacity in 1066.47 MLD, 248.71 MLD Percentage gap = 31.7% Percentage gap = 28.1% No. of Operational STPs 128 STPs + 2 FSSM No. of Operational STPs 37 No. of non-operational STPs 13 STPs No. of non-operational STPs 3 No. of Complying STPs 95 STPs complying 29 STPs complying 39 STP compliance data yet to 7 STPs compliance data yet to be procured be procured No. of Non-complying STPs 7 STPs non-complying 4 STPs non-complying 1.1 Details of each existing STP in the State: Karnataka (Annexure1a) 17 PRS (Annexure1b) Bengalur Other than Total Bengalur Other than Total u Bengaluru u Bengaluru No. of STPs 33 108 STP + 2 141STP 11 29 40 FSSM + 2 FSSM Existing treatment 1182.5 1107.06 2289.56 426 209.43 635.43 capacity (MLD) Capacity 920.6 671.78 1592.4 273.8 136.18 410 utilization (MLD) 1.2 Details of ongoing (under construction, commissioned and trial run) STPs of State: Karnataka (Annexure 2a) 17 PRS (Annexure 2b) Bengalur Other than Total Bengaluru Other than Total u Bengaluru Bengaluru Commissio No. -

Annual R Eport 201 5-1 6
Annual Report 2015-16 Contents CHAIRMAN’S LETTER ..........................................................................................................................................4 COMPANY INFORMATION .................................................................................................................................. 6 MANAGEMENT DISCUSSION AND ANALYSIS ..................................................................................................13 CORPORATE GOVERNANCE ..............................................................................................................................28 SHAREHOLDER INFORMATION ........................................................................................................................37 BOARD’S REPORT ..............................................................................................................................................49 AUDITORS’ REPORT .........................................................................................................................................102 FINANCIALS ......................................................................................................................................................106 CONSOLIDATED FINANCIAL STATEMENTS ....................................................................................................133 NOTICE .............................................................................................................................................................168 -

List of Dams in India: State Wise
ambitiousbaba.com Online Test Series List of Dams in India: State Wise State DAM and Location Rajasthan • RanapratapSagar Dam(Chambal River), at Rawatbhata • Mahi Bajaj Sagar Dam (Mahi River) at Banswara district • Bisalpur Dam (Banas River), At Tonk district • Srisailam Dam(Krishna River), at Kurnool Andhra Pradesh district • Somasila Dam (Penna River), at Nellore district • Prakasam Barrage (Krishna River), at Krishna and Guntur • Tatipudi Reservoir(River Gosthani ), at Tatipudi, Vizianagaram • Gandipalem Reservoir (River Penner) • Ramagundam dam (Godavari), in Karimnagar • Dummaguden Dam (river Godavari) Telangana • Nagarjuna Sagar Dam (Krishna river), at Nagarjuna Sagar Nalgonda • Sri Ram Sagar (River Godavari) • Nizam Sagar Dam (Manjira River) • Dindi Reservoir (River Krishna), at Dindi, Mahabubnagar town • Lower Manair Dam (Manair River) • Singur Dam (river Manjira) Bihar • Kohira Dam (Kohira River), at Kaimur district • Nagi Dam (Nagi River), in Jamui District Chhattisgarh • HasdeoBango Dam (Hasdeo River), at Korba district Gujarat • SardarSarovar Dam(Narmada river), at Navagam • Ukai Dam(Tapti River), at Ukai in Tapi district IBPS | SBI | RBI | SEBI | SIDBI | NABARD | SSC CGL | SSC CHSL | AND OTHER GOVERNMENT EXAMS 1 ambitiousbaba.com Online Test Series • Kadana Dam( Mahi River), at Panchmahal district • Karjan Reservoir (Karjan river), at Jitgadh village of Nanded Taluka, Dist. Narmada Himachal Pradesh • Bhakra Dam (Sutlej River) in Bilaspur • The Pong Dam (Beas River ) • The Chamera Dam (River Ravi) at Chamba district J & K -

Qualitative Analysis of Water of River Kali in Uttar Kannada District of Karnataka
1 EXECUTIVE SUMMARY :Project Title: QUALITATIVE ANALYSIS OF WATER OF RIVER KALI IN UTTAR KANNADA DISTRICT OF KARNATAKA All living organisms on the earth need water for their survival and growth. But due to increase in human population, industrialization, use of fertilizers in the agriculture, it is highly polluted with different harmful contaminants. Therefore, it is necessary that the quality of drinking water should be checked at regular intervals of time. Because of contaminated drinking water human populations suffers from water borne diseases. The availability of good quality water is an in dispensable feature for preventing deceases and improving quality of life. Research has proved the long term use of this savage effluent for irrigation. But this contains considerable amount of potential harmful substances including soluble salts and heavy metals. Addition of heavy metals are undesirable. So it is necessary to know details about different physico-chemical parameters such as colour, acidity, hardness, pH, sulphate, chloride DO, BOD, COD, alkalinity, etc. Some water analysis report with physico-chemical parameters have been given for exploring parameter study. 2 In general rivers are known as store house of information regarding the present and past climate, environment of pollution and degree of pollution or contamination of pollutants. It is with the background studies on physico-chemical characteristics of the water body have been conducted on the rivers the theme, Mississippi, the Amazon, the Nile. Physical and chemical methods indicates whether the pollution is of organic or inorganic origin. Although much work has been carried out on many Indian rivers and reservoirs. No detailed project work has been reported on the limnology of the river Kali of Uttar Kannada District. -

A Progress Report on Reconnaissance of Softshell Turtles in the States of Karnataka and Andhra Pradesh
A Progress Report on Reconnaissance of Softshell Turtles in the states of Karnataka and Andhra Pradesh Report Submitted to Turtle Survival Alliance & Madras Crocodile Bank Trust Under the Turtle Survival Alliance Seed Grant ( TSA-SD-IN-10-01) Duration September-October,2010 By Shashwat Sirsi Submitted on 31122010 Introduction Nilssonia leithii: Distribution: In recent years, there has been an increasing awareness of the need for positive conservation Leith’s softshell turtle is understood to be action for chelonians. However there exist, major endemic to peninsular India, reports of occurrence gaps in our knowledge of the distribution, include the Chalakudy, Bharathapuzha and ecology, behavior and status of chelonian species, Chaliyar River in Kerala (Kumar, 2004; Nameer et that are thus insufficiently known. This renders al., 2007 and Thomas et al., 1997 as cited by planning of species conservation all the more Kumar, 2004); Godavari and Krishna River from difficult but no less urgent (Conservation Action Andhra Pradesh, Cauvery and Coleroon River Plan for Endangered Freshwater Turtles and from Tamil Nadu (Kalaiarasan et al.,1992 as cited Tortoises, 2006). by Frazier and Das, 1994), Nethravathi from Karnataka, as well as Pawna in Maharashtra and All softshells in Asia are highly prized both by Godavari in Orissa (Deepak and Vasudevan, gourmands as a delicacy as well as by local 2009). communities as a source of protein. Additionally, products derived from chelonians are used in Deepak and Vasudevan (2009), delineate the traditional Chinese medicine resulting in extensive present distribution range of the species from human exploitation and consequent population Pawna (Maharashtra) as Northern and North- declines (van Dijk et al., 2000).