Nesting Ecology of Marbled Murrelets at a Remote Mainland Fjord in Southeast Alaska
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alaska Tideland Surveys
Alaska Tideland Surveys “Who, What, When, Where, How, Why” A Paper Presented at the th 37 Annual Alaska Surveying and Mapping Conference By Gerald Jennings, P.L.S., and Joe Kemmerer, P.L.S. February, 2002 State of Alaska Department of Natural Resources Division of Mining, Land and Water, Technical and Data Management 550 West 7th Ave, Suite 650 Anchorage, Alaska 99501-3576 (907) 269-8523 Fax (907) 269-8914 ii ABSTRACT Alaska Tideland Surveys – the 5 w’s. Surveys of tideland parcels are unique in several ways. Typically all corners are monumented with witness corners. DNR is usually the fee owner of the parcel, and the landward boundary is usually the mean high water line. Frequently, the line is fixed and limiting, because of avulsion, or placement of fill. This paper will briefly discuss how an applicant applies for a tideland lease or conveyance and how to conduct the survey and obtain state approval. Presenter: Gerald Jennings The Department of Natural Resources, Division of Mining, Land and Water, Technical and Data Management staff dealing with Alaska Tideland Surveys: Gerald Jennings, P.L.S., Statewide Platting Supervisor Joe Kemmerer, P.L.S., Coastal Boundary. William (Bill) Brown, P.L.S., Riparian Specialist iii Alaska Tideland Surveys Introduction – who what why? Title to most of the tide and submerged lands surrounding Alaska was vested in the State of Alaska under the Submerged Lands Act of May 22, 1953. Most of those lands remain in state ownership and in most cases, the state will lease, but retain fee title. As a surveyor, you will be contacted about Alaska Tideland Surveys (ATS) by a public or private party who desires to lease or acquire tidelands for various reasons such as construction of docks, bridges, harbors, log transfer facilities, etc. -
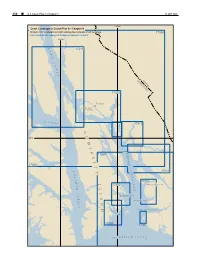
Stephens Passage
254 ¢ U.S. Coast Pilot 8, Chapter 9 19 SEP 2021 134°W Chart Coverage in Coast Pilot 8—Chapter 9 NOAA’s Online Interactive Chart Catalog has complete chart coverage 17300 http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 135°W L 17316 Y N N C A N A L UNITED STCANADA ATES 17315 T Juneau E L N DOUGLAS I ISLAND U K A T I C Y S T R A I T 17313 A 17314 D 58°N M I S R T 17360 E A P H L 17300 E T N S C Y 17311 H P A A S T S H 17363 A I A 17362 WINDHAM BAY G M S E L S T GAMBIER BAY A R A HOBART BAY N I T D PYB PORT HOUGHTON US B AY 17363 17365 I C K S O F R E D E R U N D 19 SEP 2021 U.S. Coast Pilot 8, Chapter 9 ¢ 255 Stephens Passage (1) This chapter describes Stephens Passage, Holkham during the winter, reaching its maximum in January. Bay, Endicott Arm, Tracy Arm, Taku Harbor, Gastineau The least fog occurs during April to July, inclusive, the Channel, Auke Bay, Tee Harbor and the city of Juneau, minimum being in May. including the communities of Douglas and Auke Bay. (10) (2) Ice ENC - US2AK30M (11) Ice is discharged from glaciers in Tracy and Endicott Chart - 16016 Arms, is always found in Holkham Bay and is prevalent in Stephens Passage off the entrance to that bay. -

Sweetheart Lake Hydroelectric Project—FERC Project No
20160531-3004 FERC PDF (Unofficial) 05/31/2016 FERC/EIS-0263F POA-2009-1064 FINAL ENVIRONMENTAL IMPACT STATEMENT FOR HYDROPOWER LICENSE Sweetheart Lake Hydroelectric Project—FERC Project No. 13563-003 Alaska Federal Energy Regulatory Commission Office of Energy Projects Division of Hydropower Licensing 888 First Street, NE Washington, DC 20426 U.S. Army Corps of Engineers District, Alaska Juneau Regulatory Field Office 44669B Sterling Highway Soldotna, AK 99669 May 2016 20160531-3004 FERC PDF (Unofficial) 05/31/2016 20160531-3004 FERC PDF (Unofficial) 05/31/2016 FEDERAL ENERGY REGULATORY COMMISSION WASHINGTON, D.C. 20426 OFFICE OF ENERGY PROJECTS To the Agency or Individual Addressed: Reference: Final Environmental Impact Statement Attached is the final environmental impact statement (EIS) for the proposed Sweetheart Lake Hydroelectric Project (No. 13563-003), which would be located in the City and Borough of Juneau, Alaska. This final EIS documents the view of governmental agencies, non-governmental organizations, affected Indian tribes, the public, the license applicant, and Federal Energy Regulatory Commission (Commission) staff. It contains staff evaluations of the applicant’s proposal and the alternatives for licensing the Sweetheart Lake Project. Before the Commission makes a licensing decision, it will take into account all concerns relevant to the public interest. The final EIS will be part of the record from which the Commission will make its decision. The final EIS was sent to the U.S. Environmental Protection Agency and made available to the public on or about June 10, 2016. Copies of the final EIS are available for review in the Commission’s Public Reference Branch, Room 2A, located at 888 First Street, N.E., Washington, DC 20426. -

Petersburg Borough Proposal Juneau Response Exhibit 1 Contested Area
Petersburg Borough Proposal Juneau Response Exhibit 1 Contested Area Miles Ü 05 10203040 Petersburg Boundary Proposal Juneau Contested Area Juneau Annexation Proposal Juneau Borough (current) NOTES 1. The southern boundary of the contested area, and Juneau's proposed annexation, extends to Cape Fanshaw, and is defined by the southern boundaries of the Port Houghton and Dawes Glacier watersheds. 2. Juneau's proposed annexation includes all of the contested area defined on this map, and a small portion of Stephen's Passage at the southwest corner of the current Juneau Borough boundary. October 25, 2011 -- Petersburg_Borough_Proposal_and_CBJ_response_Exhibit_1.mxd JJuunneeaauu BBoorroouugghh AAddjjuussttmmeenntt Research prepared for CBJ by JEDC 10/26/11 Table of Contents Introduction and Summary ................................................................................ 3 Administrative Boundaries.................................................................................. 5 Juneau Election District/Redistricting History ................................................ 6 Census Areas.................................................................................................. 11 Juneau Fish and Game Management Unit................................................ 13 Tongass National Forest Juneau Ranger District ........................................ 14 Model Borough Boundaries .......................................................................... 15 Juneau Recorder’s District........................................................................... -

Mineral Resources Southeastern Alaska M.I.R.L
Mineral resources of southeastern Alaska Item Type Technical Report Authors Wolff, E.N.; Heiner, L.E. Citation Wolff, E.N. and Heiner, L.E., 1971, Mineral resources of southeastern Alaska: University of Alaska Mineral Industry Research Laboratory Report No. 28, 334 p., 12 sheets. Publisher University of Alaska Mineral Industry Research Laboratory Download date 11/10/2021 10:27:19 Link to Item http://hdl.handle.net/11122/1081 MINERAL RESOURCES OF SOUTHEASTERN ALASKA M.I.R.L. Report No. 28 MINERAL INDUSTRY RESEARCH LABORATORY University of Alaska College, Alaska Ernest N . Wol ff Lawrence E. Heiner November, 1 971 TABLE OF CONTENTS Page ACKNOWLEDGMENTS i LlST OF FIGURES LlST OF TABLES LlST OF PLATES INTRODUCTION LOCATIO N BRIEF HISTORY CLIMATE PLANTS AND ANIMALS SETTLEMENTS, COMMUNICATION AND TRANSPORTATION GEOGRAPHY AND PHYSIOGRAPHY REGIONAL GEOLOGY OF THE CORDILLERA Introduction Stratigraphic Sequences and Orogenies of the Cordillera Purcell Sequence Windermere Sequence Sauk Sequence Cariboo Orogeny Cache Creek Sequence Cassiar Orogeny Hazelton-Takla Sequence Coast Range Orogeny K-rss Sequence Rocky Mountain Oregony, Late Cretaceous and Paleocene Tertiary Deposition Puget Orogeny THE TECTONIC BELTS OF THE NORTHWEST CORDILLERA DISTRIBUTION OF MINERAL DEPOSITS IN BRITISH COLUMBIA RELATION OF MINERAL DEPOSITS TO TECTONIC BELTS Page REGIONAL GEOLOGY OF SOUTHEASTERN ALASKA GENERALIZED GEOLOGIC HISTORY IGNEOUS lNTRUS I0 NS Distribution and Composition Chronology of Intrusion CORRELATION, GEOLOGY AND ORE DEPOSITS PRODUCT10 N OF MINERALS IN SOUTHEASTERN