Sarcomas of the Oral and Maxillofacial Region: Analysis of 26 Cases with Emphasis on Diagnostic Challenges
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ournal of Medicine and Health Sciences วารสารการแพทย์และวิทยาศาสตร์สุขภาพ Vol.28 No.2 August 2021
Journal of Medicine and Health Sciences วารสารการแพทย์และวิทยาศาสตร์สุขภาพ Vol.28 No.2 August 2021 https://www.tci-thaijo.org/index.php/jmhs/issue/archive ISSN 2651-1886 วารสารการแพทย์และวิทยาศาสตร์สุขภาพ (Journal of Medicine and Health Sciences) งานวิจัยและวิเทศสัมพันธ์ คณะแพทยศาสตร์ มหาวิทยาลัยศรีนครินทรวิโรฒ เวลาเผยแพร่: ก�ำหนดกำรตีพิมพ์วำรสำรฯ ปีละ 3 ฉบับ คือ ฉบับเดือน เมษำยน สิงหำคม และธันวำคม สถานที่ติดต่อ: งำนวิจัยและวิเทศสัมพันธ์ คณะแพทยศำสตร์ มหำวิทยำลัยศรีนครินทรวิโรฒ โทร: 037-395451 ต่อ 60428-9 E-mail: [email protected] เจ้าของวารสาร: ลิขสิทธิ์ของคณะแพทยศำสตร์ มหำวิทยำลัยศรีนครินทรวิโรฒ วัตถุประสงค์ เพื่อเป็นสื่อกลำงในกำรเผยแพร่ผลงำนวิจัย ผลงำนวิชำกำร และผลงำนอื่นๆ ที่เกี่ยวข้องกับกำรแพทย์ และวิทยำศำสตร์สุขภำพของคณำจำรย์ นักวิจัย นักวิชำกำร แพทย์ พยำบำล เภสัชกร ทันตแพทย์ นักกำยภำพบ�ำบัด นักวิทยำศำสตร์ บุคลำกร และนิสิตท�ำกำรศึกษำ ทั้งภำยในและภำยนอก คณะแพทยศำสตร์ มหำวิทยำลัยศรีนครินทรวิโรฒ มาตรฐาน วำรสำรกำรแพทย์และวิทยำศำสตร์สุขภำพ ผ่ำนกำรประเมินและได้รับกำรรับรองคุณภำพจำก ศูนย์ดัชนีกำรอ้ำงอิงวำรสำรไทย (Thai Journal Citation Index Center, TCI) ให้อยู่ใน TCI กลุ่มที่ 1 และ ได้กำรรับรองเข้ำสู่ฐำน Asean Citation Index (ACI) เมื่อวันที่ 24 พฤศจิกำยน 2560 ผลงำนตีพิมพ์อยู่ในรูปแบบของบทควำมวิจัย (original article) บทควำมวิจัยอย่ำงสั้น (short communication) บทควำมวิชำกำร (review article) บทควำมรำยงำนผู้ป่วย (case report) หรือ บทควำมวิชำกำรอื่นๆ ที่เกี่ยวข้องทำงกำรแพทย์และวิทยำศำสตร์สุขภำพ โดยผลงำนแต่ละเรื่องจะได้รับ กำรกลั่นกรอง (peer review) จำกผู้ทรงคุณวุฒิที่มีควำมเชี่ยวชำญหรือประสบกำรณ์ทำงกำรแพทย์และ วิทยำศำสตร์สุขภำพ ทั้งจำกภำยในและภำยนอกมหำวิทยำลัย -

Pediatric Oral Medicine
Our Speaker 15th Annual Juan F. Yepes, DDS, MD, MPH, MS, DrPH is an PEDOGATORS Associate Professor in the Department of Pediatric Continuing Education Course Dentistry at Indiana UF College of Dentistry University School of Dentistry, and Attending Faculty at Riley Hospital for Children at Indiana University in Indianapolis, Indiana. His dental and medical degrees are from Javeriana University in Bogotá, Colombia. In 1999, Dr. Yepes moved to the United States and completed a fellowship and a residency in radiology at the University of Iowa in 2002, and a residency in oral medicine at the University of Pennsylvania in 2004. In 2006 he completed a master’s degree in Public Health at the University of Kentucky and in 2008, he completed a residency in Dental Public Health at Texas A&M University, Baylor College of Dentistry. In 2011, he received a doctoral degree in Public Health with emphasis in epidemiology from the University of Kentucky, and in 2012, he completed his residency training with a master’s degree in pediatric dentistry from the University of Kentucky. Pediatric Oral Dr. Yepes is board certified by the American Boards of Pediatric Dentistry, Oral Medicine and Dental Public Medicine Health. He is an active member of the American Juan F. Yepes, DDS, MD, MPH, MS, DrPH Academy of Pediatric Dentistry, American Academy Associate Professor of Pediatric Dentistry of Oral Medicine, American Academy of Oral and Attending Faculty, Riley Hospital for Children Maxillofacial Radiology, American Association of Public Health Dentistry, Indiana Dental Association Indiana University School of Dentistry and American Dental Association. Dr. Yepes is also a Indianapolis, Indiana Fellow in Dental Surgery from the Royal College of Surgeons at Edinburgh, Scotland. -

Journal of Oral Medicine and Dental Research the COVID-19 Pathway: a Proposed Oral- Vascular-Pulmonary Route of SARS-Cov-2 I
1 Journal of Oral Medicine and Dental Research Genesis-JOMDR-2(1)-S1 Volume 2 | Issue 1 Open Access The COVID-19 Pathway: A Proposed Oral- Vascular-Pulmonary Route of SARS-CoV-2 Infection and the Importance of Oral Healthcare Measures Graham Lloyd-Jones1*, Shervin Molayem2, Carla Cruvinel Pontes3, Iain Chapple4 1Consultant Radiologist, Salisbury District Hospital, United Kingdom, Director of Radiology Masterclass 2Periodontist, Director, Mouth-Body Research Institute, Los Angeles, California 3Periodontist, Researcher, Mouth-Body Research Institute, Cape Town, South Africa 4Professor, Periodontal Research Group, Institute of Clinical Sciences, College of Medical & Dental Sciences, The University of Birmingham & Birmingham Community Health Trust, Birmingham, United Kingdom *Corresponding author: Graham Lloyd-Jones, Consultant Radiologist, Salisbury District Hospital, United Kingdom, Director of Radiology Masterclass. Citation: Lloyd-Jones G, Molayem S, Pontes CC, Copyright© 2021 by Lloyd-Jones G, et al. All rights Chapple I. (2021) The COVID-19 Pathway: A Proposed reserved. This is an open access article distributed Oral-Vascular-Pulmonary Route of SARS-CoV-2 under the terms of the Creative Commons Attribution Infection and the Importance of Oral Healthcare License, which permits unrestricted use, distribution, Measures. J Oral Med and Dent Res. 2(1):1-25. and reproduction in any medium, provided the Received: April 09, 2021 | Published: April 20, 2021 original author and source are credited. Abstract Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in late 2019, the world has faced a major healthcare challenge. There remains limited understanding of the reasons for clinical variability of coronavirus disease 2019 (COVID-19), and a lack of biomarkers to identify individuals at risk of developing severe lung disease. -

Myxoid Chondrosarcoma of Maxilla in a Pediatric Patient: a Rare Case Report
Hindawi Publishing Corporation Case Reports in Oncological Medicine Volume 2016, Article ID 5419737, 5 pages http://dx.doi.org/10.1155/2016/5419737 Case Report Myxoid Chondrosarcoma of Maxilla in a Pediatric Patient: A Rare Case Report Pranali Nimonkar,1 Nitin Bhola,1 Anendd Jadhav,1 Anuj Jain,1 Rajiv Borle,1 Rajul Ranka,2 and Minal Chaudhary2 1 Department of Oral and Maxillofacial Surgery, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra 442004, India 2Department of Oral Pathology and Microbiology, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra 442004, India Correspondence should be addressed to Anuj Jain; [email protected] Received 17 November 2015; Revised 3 January 2016; Accepted 5 January 2016 Academic Editor: Constantine Gennatas Copyright © 2016 Pranali Nimonkar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Myxoid variant of chondrosarcoma is an uncommon potentially lethal malignant tumor which is even rare in pediatric age group. In the present paper, we report one such case of intermediate grade myxoid chondrosarcoma of left side of maxilla in a 12-year- old girl. The present case had a firm, painless, and lobulated growth in premolar-molar region which was associated with bicortical expansion. Maxillofacial imaging showed ill-defined radiolucency with displaced maxillary molars. Osteolytic changes were evident with the alveolus and walls of maxillary sinus. Owing to the age of the patient, surgical excision was selected as the modality of management followed by postoperative radiotherapy. -
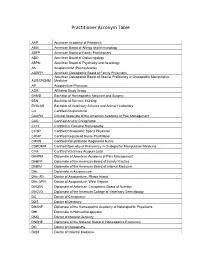
Practitioner Acronym Table
Practitioner Acronym Table AAP American Academy of Pediatrics ABAI American Board of Allergy and Immunology ABFP American Board of Family Practitioners ABO American Board of Otolaryngology ABPN American Board of Psychiatry and Neurology AK Acupuncturist (Pennsylvania) AOBFP American Osteopathic Board of Family Physicians American Osteopathic Board of Special Proficiency in Osteopathic Manipulative AOBSPOMM Medicine AP Acupuncture Physician ASG Affiliated Study Group BHMS Bachelor of Homeopathic Medicine and Surgery BSN Bachelor of Science, Nursing BVScAH Bachelor of Veterinary Science and Animal Husbandry CA Certified Acupuncturist CAAPM Clinical Associate of the American Academy of Pain Management CAC Certified Animal Chiropractor CCH Certified in Classical Homeopathy CCSP Certified Chiropractic Sports Physician CRNP Certified Registered Nurse Practitioner CRRN Certified Rehabilitation Registered Nurse CSPOMM Certified Specialty of Proficiency in Osteopathic Manipulation Medicine CVA Certified Veterinary Acupuncturist DAAPM Diplomate of American Academy of Pain Management DABFP Diplomate of the American Board of Family Practice DABIM Diplomate of the American Board of Internal Medicine DAc Diplomate in Acupuncture DAc (RI) Doctor of Acupuncture, Rhode Island DAc (WV) Doctor of Acupuncture, West Virginia DACBN Diplomate of American Chiropractic Board of Nutrition DACVD Diplomate of the American College of Veterinary Dermatology DC Doctor of Chiropractic DDS Doctor of Dentistry DHANP Diplomate of the Homeopathic Academy of Naturopathic -

Glossary of Oral and Maxillofacial Implants
gomi_frontmatter 09.08.2007 14:16 Uhr Seite III pyri Co gh Not for Publicationt b y Q u i N n o Editor-in-Chief: t t r f e o ssence W. R. Laney Glossary of Oral and Maxillofacial Implants Section Editors: N. Broggini D. Buser D. L. Cochran L. T.Garcia W. V.Giannobile E. Hjørting-Hansen T.D. Taylor Co-Editors: J. A. Cirelli K. Dula R. E. Jung R. T.Yanase Quintessence Publishing Co, Ltd Berlin, Chicago, Tokyo, Barcelona, Beijing, Istanbul, London, Milan, Moscow, New Delhi, Paris, Prague, São Paulo, Seoul, and Warsaw gomi_frontmatter 09.08.2007 14:16 Uhr Seite V pyri Co gh Not for Publicationt b y Q u i N n o t t r f e o Foreword ssence The preparation of the Glossary of Oral and Max- Implants is sure to become an indispensable illofacial Implants represents a crucial step to- tool for every professional fascinated by the vast wards harmonizing the terminology employed array of terminology in the field and who also worldwide by clinicians, researchers and aca- has the desire to employ it accurately and mean- demics who work in this field and establishing a ingfully. solid basis for mutual understanding. This volume does not aspire to the impossible The International Team for Implantology (ITI) task to cover all terms in this field. It has, how- has no hesitation in endorsing this valuable ever, selected around 2000 of the most com- work and congratulates its author, Prof. Dr. monly used terms from various areas of implant William R. -

Myxoid Chondrosarcoma of Maxilla in a Pediatric Patient: a Rare Case Report
Hindawi Publishing Corporation Case Reports in Oncological Medicine Volume 2016, Article ID 5419737, 5 pages http://dx.doi.org/10.1155/2016/5419737 Case Report Myxoid Chondrosarcoma of Maxilla in a Pediatric Patient: A Rare Case Report Pranali Nimonkar,1 Nitin Bhola,1 Anendd Jadhav,1 Anuj Jain,1 Rajiv Borle,1 Rajul Ranka,2 and Minal Chaudhary2 1 Department of Oral and Maxillofacial Surgery, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra 442004, India 2Department of Oral Pathology and Microbiology, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra 442004, India Correspondence should be addressed to Anuj Jain; [email protected] Received 17 November 2015; Revised 3 January 2016; Accepted 5 January 2016 Academic Editor: Constantine Gennatas Copyright © 2016 Pranali Nimonkar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Myxoid variant of chondrosarcoma is an uncommon potentially lethal malignant tumor which is even rare in pediatric age group. In the present paper, we report one such case of intermediate grade myxoid chondrosarcoma of left side of maxilla in a 12-year- old girl. The present case had a firm, painless, and lobulated growth in premolar-molar region which was associated with bicortical expansion. Maxillofacial imaging showed ill-defined radiolucency with displaced maxillary molars. Osteolytic changes were evident with the alveolus and walls of maxillary sinus. Owing to the age of the patient, surgical excision was selected as the modality of management followed by postoperative radiotherapy. -

Two Rare Cases of Oral Metastasis Arising from Lung Adenocarcinoma and Esophageal Carcinoma
J Clin Exp Dent. 2020;12(10):e999-1004. Two rare cases of oral metastasis Journal section: Oral Medicine and Pathology doi:10.4317/jced.57125 Publication Types: Case Report https://doi.org/10.4317/jced.57125 Two rare cases of oral metastasis arising from lung adenocarcinoma and esophageal carcinoma Breno-Amaral Rocha 1, Lívia-Maris-Ribeiro Paranaiba 2, Ciro-Dantas Soares 3, Maria-Goretti-Freire de Carvalho 4, Mário-Rodrigues de Melo-Filho 5, Lucianne-Maia-Costa Lima 6, Giovanna-Ribeiro Souto 1, Martinho-Campolina-Rebello Horta 1 1 Graduate Program in Dentistry, School of Dentistry, Pontifícia Universidade Católica de Minas Gerais (PUC Minas), Belo Hori- zonte, Minas Gerais, Brazil 2 Department of Pathology and Parasitology, Institute of Biomedical Sciences, Federal University of Alfenas, Alfenas, MG, Brazil 3 Department of Oral Diagnosis, School of Dentistry, State University of Campinas, Sao Paulo, Brazil 4 Full professor, Pathology Department, Potiguar University, Natal, Brazil 5 Dental School, University of Montes Claros, Montes Claros, Minas Gerais, Brazil 6 Radiotherapy Service, Santa Casa Hospital, Montes Claros, MG, Brazil Correspondence: Pontifícia Universidade Católica de Minas Gerais (PUC Minas) Avenida D. José Gaspar, 500. Prédio 46 – Sala 101 Belo Horizonte, Minas Gerais, Brazil. 30535901 [email protected] Rocha BA, Paranaíba LMR, Soares CD, Carvalho MGF, Melo-Filho MR, Lima LMC, Souto GR, Horta MCR. Two rare cases of oral metastasis arising from lung adenocarcinoma and esophageal carcinoma. J Clin Exp Received: 02/04/2020 Dent. 2020;12(10):e999-1004. Accepted: 02/07/2020 Article Number: 57125 http://www.medicinaoral.com/odo/indice.htm © Medicina Oral S. -

Oral Manifestations of Hepatitis B and C: a Case Series with Review of Literature
_______________________________________________________________________________CASE SERIES Oral manifestations of hepatitis B and C: A case series with review of literature Bagewadi SB1, Arora MP2, Mody BM3, Krishnamoorthy B4, Baduni A5 ABSTRACT 1,4Professor, 2,5PG student, Viral hepatitis is one of the most common liver disorders and is a major public 3Professor and HEAD, health problem occurring in almost all areas of the world. Occurrence of hepatitis Department of Oral Medicine B and C can lead to complications due to liver dysfunction, immune complex and Radiology, ITS CDSR disorders, chronic liver failure, and certain extra hepatic manifestations that can seriously affect the patient's quality of life. Awareness and recognition of these oral manifestations are of particular importance for the oral physician to facilitate early diagnosis and treatment and to take suitable measures to prevent transmission of infection. Received: 03-12-2014 Accepted: 28-03-2015 Keywords: Hepatitis B, Hepatitis C, Oral manifestations INTRODUCTION iral hepatitis is one of the most common liver CASE 1: A 40 years old female reported to the V disorders and is a major public health Department of Oral Medicine and Radiology with problem occurring in almost all areas of the chief complaint of bleeding gums since 15-20 world. The most common types of hepatitis are days. History of present illness revealed that the hepatitis A, B, C, D, E, and G. Hepatitis B Virus problem was present since 5 -6 years and (HBV) and Hepatitis C Virus (HCV) infections are increased in severity with time. Since 15-20 days serious health problems that can lead to spontaneous bleeding from gums was noticed. -

Cascade Oral Medicine Marijoyce R. Leynes, DDS, MSD Patient Health
Cascade Marijoyce R. Leynes, DDS, MSD Oral 610 Dupont St., Ste. #128; Bellingham, WA 98225 ~ Phone: 360.383.6824 ~ Fax: 888.972.9695 Medicine www.cascadeoralmed.com ~ [email protected] Inc., P.S. Patient Health History Date: Thank you for entrusting us with you care. Please complete and return this questionnaire prior to your appointment. This will help ensure our best care for you and help us understand all aspects of your health. Please answer these questions as accurately as possible. All of your information in your medical record is private and will be held confidential. General Information Last Name First Name Middle Birthdate Sex: M or F Referring Doctor: Doctor(s) you would like to be informed of your care: Chief Concern Please describe your problem: Circle any words which describe your problem: Numbness Swelling Nausea Too much / little saliva Lump or growth Ulcers Pain Altered consistency saliva Redness Blisters - Sharp Reduced or metallic taste Inflammation Yeast Infections - Dull Bad taste in mouth Infection Bleeding gums - Throbbing Strange / bad smell Burning tongue / mouth Bad breath - Electric Reduced / Increased Smell Who detected the problem? What aggravates your problem? How long have you had the problem? What alleviates symptoms? Is your problem better, worse or the same since it How often does your pain appear? (please circle) started? Constant Several times a day Daily Weekly Monthly The onset of your problem was: (please circle) Sudden Gradual Triggered by: Other: Was the onset of your symptoms related to -

Visfatin Promotes the Metastatic Potential of Chondrosarcoma Cells by Stimulating AP-1-Dependent MMP-2 Production in the MAPK Pathway
International Journal of Molecular Sciences Article Visfatin Promotes the Metastatic Potential of Chondrosarcoma Cells by Stimulating AP-1-Dependent MMP-2 Production in the MAPK Pathway Shih-Ya Hung 1,2,† , Chih-Yang Lin 3,†, Cheng-Chieh Yu 4, Hsien-Te Chen 5,6, Ming-Yu Lien 7,8, Yu-Wen Huang 4, Yi-Chin Fong 5,9, Ju-Fang Liu 10, Shih-Wei Wang 11,12,13 , Wei-Cheng Chen 11,14,* and Chih-Hsin Tang 3,4,15,16,* 1 Graduate Institute of Acupuncture Science, China Medical University, Taichung 404022, Taiwan; [email protected] 2 Department of Medical Research, China Medical University Hospital, Taichung 404022, Taiwan 3 Department of Pharmacology, School of Medicine, China Medical University, Taichung 404022, Taiwan; [email protected] 4 Graduate Institute of Biomedical Sciences, China Medical University, Taichung 404022, Taiwan; [email protected] (C.-C.Y.); [email protected] (Y.-W.H.) 5 Department of Sports Medicine, College of Health Care, China Medical University, Taichung 404022, Taiwan; [email protected] (H.-T.C.); [email protected] (Y.-C.F.) 6 Department of Orthopedic Surgery, China Medical University Hospital, Taichung 404022, Taiwan 7 Division of Hematology and Oncology, Department of Internal Medicine, China Medical University Hospital, Taichung 404022, Taiwan; [email protected] 8 Graduate Institute of Basic Medical Science, China Medical University, Taichung 404022, Taiwan 9 Department of Orthopedic Surgery, China Medical University Beigang Hospital, Yunlin 651012, Taiwan 10 School of Oral Hygiene, College of Oral Medicine, Taipei Medical University, Taipei 110301, Taiwan; Citation: Hung, S.-Y.; Lin, C.-Y.; Yu, [email protected] C.-C.; Chen, H.-T.; Lien, M.-Y.; Huang, 11 Department of Medicine, MacKay Medical College, New Taipei City 252005, Taiwan; [email protected] Y.-W.; Fong, Y.-C.; Liu, J.-F.; Wang, 12 Graduate Institute of Natural Products, College of Pharmacy, Kaohsiung Medical University, S.-W.; Chen, W.-C.; et al. -

ODM Oral Diagnosis and Oral Medicine
College of Dentistry ODM Oral Diagnosis and Oral Medicine ODM 810 BASIC PRINCIPLES IN ORAL AND MAXILLOFACIAL RADIOLOGY. (2) This course presents the basic principles of oral and maxillofacial radiology, including radiation biology, radiation physics and Imaging Principles, radiation protection and safety, and radiology techniques. Prereq: Admission to the College of Dentistry. ODM 814 ORAL DIAGNOSIS/ORAL MEDICINE AND TREATMENT PLANNING. (2) This course is designed to introduce and prepare the student dentists to better understand the importance of patient evaluation including the acquisition of the medical history, dental history, social history and family history. Students will be provided information that leads to an understanding of the significance of a proper head and neck examination, intraoral examination and oral cancer screening. Teachings and learning resources will introduce students to an initial understanding of the different needs and modifications required for certain patient population (pediatric, geriatrics, special needs and medically complex patients). This course will consist of lectures, simulated case presentations, hands on clinical examination training in groups, self-practice time and handouts. Prereq: 1st Year UKCD student. ODM 820 ORAL AND MAXILLOFACIAL RADIOLOGY AND DIAGNOSTIC IMAGING. (2) This course presents the principles of radiographic anatomy, extra-oral projections (including panoramic film and lateral skull film), radiology of caries and periodontal disease, digital radiology, advanced imaging techniques (including CBTC), and the process of radiographic interpretation. Prereq: ODM 810. ODM 821 CLINICAL ORAL DIAGNOSIS I. (1) This course consists of two components: 1) examination, diagnosis, and treatment planning for patients assigned to dental students in general clinics; and 2) an emergency clinic assignment in which the students will diagnose and treat patients with acute oral problems.