Advancing Frontiers in Bone Bioprinting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
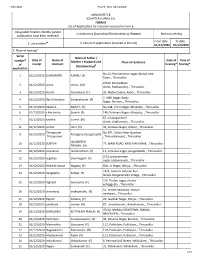
ANNEXURE 5.8 (CHAPTER V, PARA 25) FORM 9 List of Applica Ons For
11/21/2020 Form9_AC2_02/12/2020 ANNEXURE 5.8 (CHAPTER V, PARA 25) FORM 9 List of Applicaons for inclusion received in Form 6 Designated locaon identy (where Constuency (Assembly/£Parliamentary): Ponneri Revision identy applicaons have been received) From date To date 1. List number@ 2. Period of applicaons (covered in this list) 01/12/2020 01/12/2020 3. Place of hearing* Serial Name of Father / number$ Date of Name of Date of Time of Mother / Husband and Place of residence of receipt claimant hearing* hearing* (Relaonship)# applicaon No. -
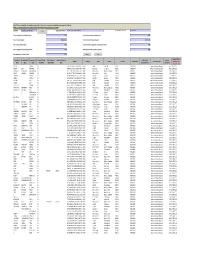
CIN/BCIN Company/Bank Name Date of AGM(DD-MON-YYYY)
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e-form IEPF-2 CIN/BCIN L67120RJ1951PLC045966 Prefill Company/Bank Name JK TYRE & INDUSTRIES LIMITED Date Of AGM(DD-MON-YYYY) 02-SEP-2016 Sum of unpaid and unclaimed dividend 0.00 Sum of interest on matured debentures 0.00 Sum of matured deposit 10986784.00 Sum of interest on matured deposit 2652436.00 Sum of matured debentures 0.00 Sum of interest on application money due for refund 0.00 Sum of application money due for refund 0.00 Redemption amount of preference shares 0.00 Sales proceed for fractional shares 0.00 Validate Clear Proposed Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id-Client Id- Amount Address Country State District Pin Code Folio Number Investment Type transfer to IEPF Name Name Name First Name Middle Name Name Account Number transferred (DD-MON-YYYY) KANTA SINGHAL NA C-175 SURAJ MAL VIHAR DELHI INDIA Delhi East Delhi 110092 P00149014 Interest on matured deposits 285 11-SEP-2016 PADMA DEVI MAKHARIA NA WARD NO.6/38 PO. PILANI PILANI RAJASTHANINDIA Rajasthan Jhunjhunu 333031 P00140944 Amount for matured deposits 460 12-SEP-2016 GUNVANTI JHAMATMAL MIRCHANDANI NA 89 GR FLOOR SINDHUWADI GHATKOPARINDIA EAST MUMBAI MaharashtraMAHARASHTRA Mumbai Suburban 400077 P00142829 Interest on matured deposits 208 14-SEP-2016 DINKER MANGESH MUJMDAR NA SR.156 PLOTNO.15 GANESHNO.2 OPP.SHIVAINDIA MANDIR (VISHWESHWAR Maharashtra )BIJALI NAGAR Pune CHINCHWAD PUNE MAHARASHTRA 411033 P00134078 Interest on matured deposits 189 19-SEP-2016 LOVELEEN KHANNA NA C/O MRS MADHU KOHLI C-2597 SUSHANTINDIA LOK GURGAON Haryana HARYANA Gurgaon 122009 P00132279 Amount for matured deposits 177 19-SEP-2016 AZEEZA SULTANA NA DOOR NO.196 K.E.B. -
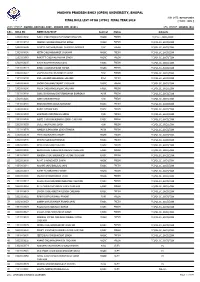
Madhya Pradesh Bhoj (Open) University, Bhopal
MADHYA PRADESH BHOJ (OPEN) UNIVERSITY, BHOPAL RUN DATE: 03-Oct-2019 FINAL ROLL LIST OF BA (3YDC) FINAL YEAR 2019 [ MODE : ODL ] STUDY CENTRE : RGMMC, BAKTARA, DIST.- SEHORE (MP) (0101) REG. CENTRE : BHOPAL (01) S.No. ROLL NO NAME/S/D/W OF Sex/Cat Status Subjects g 1 13010108138 AJEET CHOUHAN/RUKHAM SINGH CHOUHAN M/OBC FRESH FC,H.LIT.,SOCI,ECON 2 14010108263 DEEPAK CHOUHAN/NARAYAN SINGH M/OBC FRESH FC,POL.SC.,SOCI,ECON 3 14010108296 NAMITA AHIRWAR/PHOOL CHANDRA AHIRWAR F/SC FRESH FC,POL.SC.,SOCI,ECON 4 15010108036 NITIN CHOUHAN/BHAIJI CHOUHAN M/OBC FRESH FC,POL.SC.,SOCI,ECON 5 15010108063 RANJEET CHOUHAN/MOHAN SINGH M/OBC FRESH FC,POL.SC.,SOCI,ECON 6 15010108105 KIRAN KUSHWAHA/BALAKDAS F/OBC FRESH FC,POL.SC.,SOCI,ECON 7 15010108114 AMAN CHOUHAN/PAVAN KUMAR M/OBC FRESH FC,POL.SC.,SOCI,ECON 8 15010108217 LAXMINARAYAN NA/HANMAT SINGH F/SC FRESH FC,POL.SC.,SOCI,ECON 9 15010108230 ANIL SOLANKI/LEELADHAR SOLANKI M/SC FRESH FC,POL.SC.,SOCI,ECON 10 15010108232 SAGAR CHOUHAN/BASANT CHOUHAN M/OBC FRESH FC,POL.SC.,SOCI,ECON 11 15010108266 PRIYA CHOUHAN/SURESH CHOUHAN F/OBC FRESH FC,POL.SC.,SOCI,ECON 12 15010108268 SONU BHARGAVA/CHATURNARAYAN BHARGAVA M/UR FRESH FC,POL.SC.,SOCI,ECON 13 15010108281 SONA XXX/KANCHHEDI F/SC FRESH FC,POL.SC.,SOCI,ECON 14 15010108285 BHAGYASHREE XXXX/DHANSINGH F/OBC FRESH FC,POL.SC.,SOCI,ECON 15 15010108331 RAJNI X/PHOOLWAN F/OBC FRESH FC,POL.SC.,SOCI,ECON 16 15010108333 SANJIWAN XXXX/MADHO SINGH F/UR FRESH FC,POL.SC.,SOCI,ECON 17 15010108340 AARTI CHOUHAN/KUNWAR SINGH CHOUHAN F/OBC FRESH FC,POL.SC.,SOCI,ECON 18 15010108355 -

STAMPS of INDIA COLLECTORS COMPANION ------The First & Only Weekly on Philately & Postal Services of India
ISSN 0972-3587 -------------- STAMPS OF INDIA COLLECTORS COMPANION --------------- The First & Only Weekly on Philately & Postal Services of India Issue # 257 – January 26, 2006. Published Every Thursday Edited by Madhukar and Savita Jhingan ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ I N T H I S I S S U E Forthcoming Stamp Issues AVM Stamp Released Postal Stationary New Issues Recent Special Postmarks & Covers Blood Donation Camp at Dehradun HPO Packaging Cartons at Post Office Instant Money Order Recent & Forthcoming Events Nepal Program 2006 New Commemorative Coins Errors Freaks & Oddities: Agra Fort Stamps ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ To SUBSCRIBE, send email giving your name, postal address, and philatelic interests to [email protected] To UNSUBSCRIBE, send email to [email protected] The BACK ISSUES are available as Printout, on CD, and on line at http://www.stampsofindia.com/newssite/Download/archives.htm ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ JHINGANS JOTTINGS Hi This year completes 150 years of India’s Field Post Offices. The first Field Post Office, then called Field Force Post Office, accompanied the Expeditionary Forces to Persia (now Iran) under the command of Major General Sir James Outram. An Indian naval squadron commanded by Commodore Young was also a part of this Force that arrived at Bushire on December 5, 1856 where the Persian Field Force Post Office functioned from December 12, 1856 to October 2, 1857. This Field Force Post Office’s use of postage stamps was the first instance of postage stamps used in Persia. The first stamps issued by Persia were actually in 1875 although stamps were also issued in 1868 but believed to have not been circulated to public, and again in 1870 for a short while and their use was ceased in 1871. -

Officials from the State of Tamil Nadu Trained by NIDM During the Year 209-10 to 2014-15
Officials from the state of Tamil Nadu trained by NIDM during the year 209-10 to 2014-15 S.No. Name Designation & Address City & State Department 1 Shri G. Sivakumar Superintending National Highways 260 / N Jawaharlal Nehru Salai, Chennai, Tamil Engineer, Roads & Jaynagar - Arumbakkam, Chennai - 600166, Tamil Nadu Bridges Nadu, Ph. : 044-24751123 (O), 044-26154947 (R), 9443345414 (M) 2 Dr. N. Cithirai Regional Joint Director Animal Husbandry Department, Government of Tamil Chennai, Tamil (AH), Animal husbandry Nadu, Chennai, Tamil Nadu, Ph. : 044-27665287 (O), Nadu 044-23612710 (R), 9445001133 (M) 3 Shri Maheswar Dayal SSP, Police Superintending of Police, Nagapattinam District, Tamil Nagapattinam, Nadu, Ph. : 04365-242888 (O), 04365-248777 (R), Tamil Nadu 9868959868 (M), 04365-242999 (Fax), Email : [email protected] 4 Dr. P. Gunasekaran Joint Director, Animal Animal Husbandary, Thiruvaruru, Tamil Nadu, Ph. : Thiruvarur, Tamil husbandary 04366-205946 (O), 9445001125 (M), 04366-205946 Nadu (Fax) 5 Shri S. Rajendran Dy. Director of Department of Agriculture, O/o Joint Director of Ramanakapuram, Agriculture, Agriculture Agriculture, Ramanakapuram (Disa), Tamil Nadu, Ph. : Tamil Nadu 04567-230387 (O), 04566-225389 (R), 9894387255 (M) 6 Shri R. Nanda Kumar Dy. Director of Statistics, Department of Economics & Statistics, DMS Chennai, Tamil Economics & Statistics Compound, Thenampet, Chennai - 600006, Tamil Nadu Nadu, Ph. : 044-24327001 (O), 044-22230032 (R), 9865548578 (M), 044-24341929 (Fax), Email : [email protected] 7 Shri U. Perumal Executive Engineer, Corporation of Chennai, Rippon Building, Chennai - Chennai, Tamil Municipal Corporation 600003, Ph. : 044-25361225 (O), 044-65687366 (R), Nadu 9444009009 (M), Email : [email protected] National Institute of Disaster Management (NIDM) Trainee Database is available at http://nidm.gov.in/trainee2.asp 58 Officials from the state of Tamil Nadu trained by NIDM during the year 209-10 to 2014-15 8 Shri M. -

List of Faculties for AICTE Approved Institutions (A.Y. 2015-16) As on 30Th April 2015
List of Faculties for AICTE approved institutions (A.Y. 2015-16) as on 30th April 2015 FACULTY DATE OF INSTITUTE SR. NO. SURNAME FIRST NAME INSTITUTE ID STATE PROGRAMME ID BIRTH NAME ACHARIYA COLLEGE OF ENGINEERING AND 1 1-2185930143 REVATHI REVATHI 19-Nov-80 1-2451494382 Puducherry ENGINEERING TECHNOLOGY TECHNOLOGY ACHARIYA COLLEGE OF ENGINEERING AND 2 1-2186006922 ARUN ARUN 11-Jun-88 1-2451494382 Puducherry ENGINEERING TECHNOLOGY TECHNOLOGY ACHARIYA COLLEGE OF ENGINEERING AND 3 1-2187191682 P.JESSY P.JESSY 07-Jul-67 1-2451494382 Puducherry ENGINEERING TECHNOLOGY TECHNOLOGY ROBIN ROBIN ACHARIYA COLLEGE OF ENGINEERING AND 4 1-2187231941 01-Jun-91 1-2451494382 Puducherry MARLAR.R MARLAR.R ENGINEERING TECHNOLOGY TECHNOLOGY ACHARIYA COLLEGE OF ENGINEERING AND 5 1-2187231948 MURUGAN VINOTH KUMAR 03-May-90 1-2451494382 Puducherry ENGINEERING TECHNOLOGY TECHNOLOGY ACHARIYA COLLEGE OF ENGINEERING AND 6 1-2187232335 PUNITHA.D PUNITHA.D 21-Jun-83 1-2451494382 Puducherry ENGINEERING TECHNOLOGY TECHNOLOGY ACHARIYA COLLEGE OF ENGINEERING AND 7 1-2187361990 V SARANYA V SARANYA 24-Dec-90 1-2451494382 Puducherry ENGINEERING TECHNOLOGY TECHNOLOGY V.S.MAHADEVA V.S.MAHADEVA ACHARIYA COLLEGE OF ENGINEERING AND 8 1-2187429139 26-Oct-87 1-2451494382 Puducherry N N ENGINEERING TECHNOLOGY TECHNOLOGY ACHARIYA COLLEGE OF ENGINEERING AND 9 1-2187480019 M.AMUDHAN M.AMUDHAN 22-Sep-88 1-2451494382 Puducherry ENGINEERING TECHNOLOGY TECHNOLOGY ACHARIYA COLLEGE OF ENGINEERING AND 10 1-2187544707 P.SIVASANKARI P.SIVASANKARI 05-Aug-90 1-2451494382 Puducherry ENGINEERING -

Dated : 23/4/2016
Dated : 23/4/2016 Signatory ID Name CIN Company Name Defaulting Year 02700070 PARATE VIJAYKUMAR U45200MH1993PTC072352 PARATE DEVELOPERS P LTD 2008-09, 2009-10 02700109 NATESAN RAMACHANDRAN U51505TN2002PTC049271 RESHMA ELECTRIC PRIVATE 2009-10 LIMITED 02700110 JEGADEESAN MAHENDRAN U51505TN2002PTC049271 RESHMA ELECTRIC PRIVATE 2009-10 LIMITED 02700187 KUMARASWAMY KUNIGAL U93090KA2006PLC039899 EMERALD AIRLINES 2009-10 RAMACHANDRA LIMITED 02700226 HENDIN URI ZIPORI U55101HP2008PTC030910 INNER WELLSPRING 2009-10 HOSPITALITY SERVICES 02700285 DEVADASON NALLATHAMPI U72200TN2006PTC059044 ZENTERE SOLUTIONS 2007-08, 2008-09, 2009-10 PRIVATE LIMITED 02700394 MENON PADMINI U72200KL2000PTC013771 E BIZ SOFTWARE PRIVATE 2006-07, 2007-08 LIMITED 02700444 GEETHA PITCHAIMANI U45201TN2005PTC057648 RAJTILAK BUILDERS 2009-10 PRIVATE LIMITED 02700472 AVDHESH R VERMA U51909MH2008PTC177604 NIDDHISH IMPEX PRIVATE 2008-09, 2009-10 LIMITED 02700521 JOSE THOMAS U70101KL2007PTC020141 SEVEN SEAS PROPERTIES 2008-09 PRIVATE LIMITED 02700550 PARGAT SINGH U64202KA2003PTC031601 BYOND GLOBAL 2009-10 OUTSOURCING PRIVATE 02700951 AGRAWAL ANJITA U20103MP1998PTC012579 VAIBHAV INDUSTRIES 2009-10 PRIVATE LIMITED 02701013 JAIN KUMAR MAHENDRA U74140MH2008PTC184622 WARBURG TEMPLIN 2009-10 ADVISORS PRIVATE LIMITED 02701127 PRASAD SEEMA U02212MP1974PTC001273 BOMBAY DRUGS BIOCARE 2009-10 PRIVATE LIMITED 02701131 AMIT KAUNDAL U52599HP2002PTC025402 SUNNET NETWORK 2006-07, 2007-08, 2009-10 SERVICES PRIVATE LIMITED 02701152 KUMAR MANOJ U52599HP2002PTC025402 SUNNET NETWORK 2006-07, 2007-08, -

Dictionary of Martyrs India's Freedom Struggle
DICTIONARY OF MARTYRS INDIA’S FREEDOM STRUGGLE (1857-1947) ii Dictionary of Martyrs: India’s Freedom Struggle (1857-1947) Vol. 2, Part II(L-Z) DICTIONARY OF MARTYRSMARTYRS INDIA’S FREEDOM STRUGGLE (1857-1947) Vol. 2 Uttar Pradesh, Uttarakhand, Madhya Pradesh, Chhattisgarh, Rajasthan and Jammu & Kashmir (1857-1947) Part II (L-Z) General Editor Basudev Chatterji Chairman, ICHR Executive Editor Ishrat Alam Research Consultant Amit Kumar Gupta Research and Editorial Team Ashfaque Ali Rajesh Kumar Md. Naushad Ali Kh. Premjit Singh Published by INDIAN COUNCIL OF HISTORICAL RESEARCH in association with MANAK PUBLICATIONS PVT. LTD iv Dictionary of Martyrs: India’s Freedom Struggle (1857-1947) Vol. 2, Part II(L-Z) Project of INDIAN COUNCIL OF HISTORICAL RESEARCH and MINISTRY OF CULTURE, GOVERNMENT OF INDIA First Edition 2013 Published by INDIAN COUNCIL OF HISTORICAL RESEARCH 35, FEROZESHAH ROAD, NEW DELHI - 110 001 in association with MANAK PUBLICATIONS PVT. LTD B-7, Saraswati Complex, Subhash Chowk, Laxmi Nagar, New Delhi 110092 INDIA Phone: 22453894, 22042529 Email: [email protected] [email protected] USA Office 8145 KOLB AVE, ALLEN PARK, M.I. 48101 USA Email: [email protected] All rights reserved © ICHR, 2013 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. ISBN 978-93-7831-338-7 (Part I) ISBN 978-93-7831-339-4 (Part II) Laser Typeset by TABREZ ALI, Laxmi Nagar, New Delhi Printed in India by Nice Printing Press, New Delhi FROM THE GENERAL EDITOR I have great pleasure in placing before the reading public Part I [A to K] and Part II [L to Z] of Volume 2 of the Dictionary of Martyrs. -

List of Applicants Who Have Applied Under CLSS Transfered to HDFC Bank
List of applicants who have applied under CLSS transfered to HDFC Bank. Sr_no Name Father_Name House_No Street_Slum 1 AADESH MADHO RAM SEC-52 2 AAISHA MOHD. SINU 675/2 SECTOR 38 A 3 AAKANSHA SK SINGLA 2233 STAR ENCLAVE SOCIETY 48 4 AAKASH LT. ASHOK KUMAR 2323 DADU MAJRA COLONY 5 Aakash Subrato Chakraborty 981A 47 6 AAMIR KHAN BUNDU HASSAN 2558, PHASE 2 RAMDARBAR 7 AANCHAL RAJENDRA KUMAR 2562 38 C 8 AARTI MANOJ 138 KHUDA JASSU 9 AARTI VINOD KUMAR 2227 SECTOR 38 (WEST),DMC 10 AARTI BENWAL GIANI RAM BENIWAL 2009-B SECTOR 41 C 11 AARTI BHARDWAJ DK BHARDWAJ 2830/2 49 12 AARTI DEVI MUNNA LAL 9/18,2ND FLOOR ATTAWA 13 AARTI DEVI RANJIT 495 25-D 14 AARTI GUPTA VEER BAHADUR 42-B 15 AARTI RAI NANAK CHAND 613, PHASE 2 RAMDARBAR 16 AARTI RANI OM SINGH 1679 SECTOR 38 (WEST),DMC 17 AARTI SHARMA ARJUN LAL SHARMA 564 KILA GATE , MANIMAJRA 18 AASHI LT ANIL KUMAR 2311/2 SEC-45/C 19 AASTHA RANI AJAY KUMAR 218 SECTOR 41 A 20 ABBAS ANSARI CHULAHAI ANSARI 1037 29 B 21 ABBDUL HAKIM MOHAMMAD HARIF 159 PANDIT COLONY VILLAGE KAJHERI 52 22 ABDUL HAMID RAHIM MIYAN 1171, PHASE 2 RAMDARBAR 23 ABDUL KUDDUS ABDUL AZIZ 2068, BURAIL 24 ABDUL RAHIM ABDUL JABBAR 1445 29 B 25 ABDUL REHMAN RAIS AHMED 3738, MALOYA 39 26 ABDUL SATTAR SAMEER AHMED 40, BURAIL 45 27 ABDUL SULTAN ABDUL MAJID 1161, BURAIL 28 ABDULLAH NASEEM AHMED 1426 MANIMAJRA 29 ABHIJIT JANA ARVIND JANA 2694 DADU MAJRA COLONY 30 ABHINASH PRASHAR AMAR SINGH 149 A KHUDA LAHORA 31 ABHINAV AGGARWAL KUL BHUSHAN GOEL 2193 49 32 ABHINAV BHARDWAJ RAKESH CHAND 3073 SEC 40 List of applicants who have applied under CLSS transfered to HDFC Bank. -

Akhtar Jawad - Poems
Poetry Series Akhtar Jawad - poems - Publication Date: 2015 Publisher: Poemhunter.com - The World's Poetry Archive Akhtar Jawad(8-2-1945) I was born on 8th of February,1945 at 8 AM www.PoemHunter.com - The World's Poetry Archive 1 3d Computer Program A great scientist on his flying horse, Orbiting in a very big elliptical orbit, Once upon a time came very close, To my insignificant too small orbit, My small path he almost touched, Remained for quiet some times, In touch and in love as well, Took samples of my soil, Took samples of my air, Took samples of my fire, Took samples of my water, And finally samples of my light. He is a great artist, Painted a picture of mine, On a white canvas of love, And when I delivered a child, He took my child, To a beautiful garden, A garden of flowers, And the fruits, But my son was alone, All praise to the cloning, His mate was created. My son tasted the pleasant fruit, Pre-matured and before the time, And they were sent back, To me once again! With pains and blood, And undesired death! And when ugliness blackened my face, He sent his robots, At my thoughtful soil, The autumn was changed, In a spring for some time, His voice messages, Were played on flutes, www.PoemHunter.com - The World's Poetry Archive 2 In the bells that ring, At dawn and dusk, And in the loud human voices, All the tunes inspire to love, And paint the beauty, Of the great scientist! Now he is too far, In a path out of reach, The robots don't come, But the loving scientist, Expects from the two, Now matured enough, To keep my face, Neat and clean, Green and fertile, And let evolution, To travel on a path, That leads to a land, Where death is dead, I am aware of success, I am aware of failures, I am a mother, I know my children. -

Transfered to IEPF
SATHAVAHANA ISPAT LIMITED UNPAID DIVIDEND DATA FOR THE YEAR 2009-10 Dividend SLNO FOLIONO NAME OF THE SHARE HOLDER SHARE HOLDER ADDRESS PIN No. of Shares Amount (in Rs.) 1 19204 A ANUSOOYA H NO 7 NORTH CAR STREET RAMESWARAM RAMNAD DIST RAMESWARAM 623526 100 150.00 2 IN30051310356402 A ARUNA 17 71/1 RAJA MEDICALS B KOTHAKOTA POST CHITTOOR DIST 517370 200 300.00 3 IN30102221302854 A B R K VARA PRASAD D NO 1 141 DWAJASTAMBAM STREET PALANGI POST UNDRAJAVARAM MANDAL 534216 20 30.00 4 32168 A B S MANIAM 25, VEERASAMY STREET GROUND FLOOR PURASAWALKAM CHENNAI 600007 100 150.00 5 IN30286310222329 A BHUPATHI RAO 39-84/85 BANDAR NAGAR WANAPARTHY MAHABOOB NAGAR 509103 500 750.00 6 6153 A C CHANDY 4/604 TULSI DHAM MAJIWADA PO THANE THANE 400607 100 150.00 7 IN30177410302360 A CHANDRAN G 9 TAC NAGAR TUTICORIN 628008 1 2.00 8 39588 A GANESH 61, EGMORE HIGH ROAD EGMORE CHENNAI 600008 100 150.00 9 18768 A JAYA PRAKASH M-191 IX TH CROSS ST 3RD MAIN RD T V R NAGAR THIRUVANMIYUR CHENNAI 600041 200 300.00 10 IN30290241783218 A K CHORDIA 61 SANJEEVAPPA COLONY 1ST FLOOR JAI BHARATH NAGAR BANGALORE 560033 350 525.00 11 18949 A K SOBHANA SEKHAR W/O C K SEKHARAN PLOT 16 D NO 10 NARMADA NAGAR GAJALAKSHMI ST ULLKARAM CHENNAI 600091 100 150.00 12 34892 A K VASUMATHY B1/51, VASUNDARA APARTMENTS S V ROAD BORIVILI (W) MUMBAI 400092 100 150.00 13 27572 A KUMAR TYPE II/11B, TATA COLONY, AZIZ BAUG CHEMBUR BOMBAY 400074 100 150.00 14 18143 A L NAVEEN 40/A 65 MAIN 11TH CROSS 3RD PHASE J P NAGAR BANGALORE 560078 100 150.00 15 26068 A LAKSHMI KANTHAM MAMATA APARTMENTS(A2) -
Paid Q1 Rajepur Rural.Xlsx
Serial No. Block Name Panchayat Name Village Name Name father husband Name Age Gender Category Name Mobile Number 1 RAJEPUR AASAMPUR UGARPUR SONELAL BHOLA NATH 70 M GENERAL 0 2 RAJEPUR AASAMPUR UGARPUR SUBEDAR KHURAM SINGH 69 M OBC 3 RAJEPUR AATAR ANTAR BADLE LALMAN 72 M SC 9450697768 4 RAJEPUR AATAR ANTAR CHOTI BITIYA CHOTE SINGH 69 F GENERAL 7860950492 5 RAJEPUR AATAR ANTAR JAVITRI RADHISYAM 66 F OBC 6 RAJEPUR AATAR ANTAR LALA RAM RATIRAM 64 M OBC 9450697768 7 RAJEPUR AATAR ANTAR MUNNI DEVI LALLA SINGH 64 F GENERAL 8 RAJEPUR AATAR ANTAR NIRMALA DEVI SONPAL 65 F OBC 0 9 RAJEPUR AATAR ANTAR RADHAESYAM MAUJI LAL 65 M OBC 9450697768 10 RAJEPUR AATAR ANTAR RAM BABU RAMSEWAK 63 M GENERAL 11 RAJEPUR AATAR ANTAR RAM NATH SINGH MACHAL SINGH 68 M GENERAL 12 RAJEPUR AATAR ANTAR RAMPAL SINGH CHHATRAPAL 69 M GENERAL 13 RAJEPUR AATAR ANTAR RAMPIYARI SOVRAN 72 F OBC 9450697768 14 RAJEPUR AATAR ANTAR RAMPOTHI JAIRAM 70 F OBC 9450697768 15 RAJEPUR AATAR ANTAR SHIRI RAM KADILE 69 M OBC 16 RAJEPUR AATAR ANTAR SONPAL PARSADI 69 M OBC 17 RAJEPUR AATAR ANTAR VISHVA NATH BHOLA NATH 72 M GENERAL 9450697768 18 RAJEPUR AATAR DEOSI HUSAINPUR RAM LADAITE MAIKA 66 M OBC 19 RAJEPUR ALIGARH MOHADDIN NAGAR ALLIGARH AFROJI BEGAM BUDHHAN KHAN 72 F MINORITY 20 RAJEPUR ALIGARH MOHADDIN NAGAR ALLIGARH CHAND PAL RAM SINGH 72 M OBC 21 RAJEPUR ALIGARH MOHADDIN NAGAR ALLIGARH DAYARAM BHOOP RAM 71 M OBC 22 RAJEPUR ALIGARH MOHADDIN NAGAR ALLIGARH HARI BABU RAM CHARAN 72 M OBC 23 RAJEPUR ALIGARH MOHADDIN NAGAR ALLIGARH JADUNATH CHITAN 72 M OBC 24 RAJEPUR ALIGARH MOHADDIN NAGAR