ATRA and Arsenic Trioxide, Give Dexamethasone (10Mg Bd IV) Until Symptoms Resolve (See Below)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ATRA) with Arsenic Trioxide (ATO) Induction Therapy
NCCP Chemotherapy Regimen Tretinoin (ATRA) with Arsenic Trioxide (ATO) Induction Therapy INDICATIONS FOR USE: Regimen *Reimbursement INDICATION ICD10 Code Status Treatment of patients with newly diagnosed low to intermediate C92 00356a Hospital risk Acute Promyelocytic Leukaemia (APL) *If the reimbursement status is not defined i, the indication has yet to be assessed through the formal HSE reimbursement process. TREATMENT: The starting dose of the drugs detailed below may be adjusted downward by the prescribing clinician, using their independent medical judgement, to consider each patients individual clinical circumstances. The treatment is administered until haematological complete remission (CR) or for a maximum of 60 days. Patients who achieve haematological CR progress to Consolidation Therapy with all trans retinoic acid (ATRA) and Arsenic Trioxide (Reference NCCP Regimen 00357). CR is defined as where the bone marrow is regenerating normal haematopoietic cells and contains <5% blast cells by morphology in an aspirate sample with at least 200 nucleated cells. Day Drug Dose Route Cycle 1 until complete Tretinoin 45mg/m2 in aPO n/a Continuous until remission (ATRA) divided doses Complete Remission (CR) is achieved or up to a maximum of 60 days 1-5 inclusive Arsenic 0.3mg/kg IV 250ml of 0.9% NaCl over Week 1 only trioxide infusion 2 hoursb Twice weekly Arsenic 0.25mg/kg IV 250ml of 0.9% NaCl over Week 2 to 8 trioxide infusion 2 hoursb 1 until end of Prednisolone 0.5mg/kg/day PO induction a Tretinoin (ATRA) is available as 10mg capsules. Round dose to nearest 10mg. The capsules should be swallowed whole with water. -

Acitretin; Adapalene; Alitretinoin; Bexarotene; Isotretinoin
8 February 2018 EMA/254364/2018 Pharmacovigilance Risk Assessment Committee (PRAC) Assessment report Referral under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data Retinoids containing medicinal products INN: Acitretin, Adapalene, Alitretinoin, Bexarotene, Isotretinoin, Tretinoin, Tazarotene Procedure number: EMEA/H/A-31/1446 Panretin EMEA/H/A-31/1446/C/000279/0037 Targretin EMEA/H/A-31/1446/C/000326/0043 Note: Assessment report as adopted by the PRAC and considered by the CHMP with all information of a commercially confidential nature deleted. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ......................................................................................... 2 1. Information on the procedure ................................................................. 3 2. Scientific discussion ................................................................................ 3 2.1. Introduction......................................................................................................... 3 2.2. Clinical aspects .................................................................................................... 5 2.3. Data on efficacy .................................................................................................. -

Aetna Formulary Exclusions Drug List
Covered and non-covered drugs Drugs not covered – and their covered alternatives 2020 Advanced Control Plan – Aetna Formulary Exclusions Drug List 05.03.525.1B (7/20) Below is a list of medications that will not be covered without a Key prior authorization for medical necessity. If you continue using one of these drugs without prior approval, you may be required UPPERCASE Brand-name medicine to pay the full cost. Ask your doctor to choose one of the generic lowercase italics Generic medicine or brand formulary options listed below. Preferred Options For Excluded Medications1 Excluded drug name(s) Preferred option(s) ABILIFY aripiprazole, clozapine, olanzapine, quetiapine, quetiapine ext-rel, risperidone, ziprasidone, VRAYLAR ABSORICA isotretinoin ACANYA adapalene, benzoyl peroxide, clindamycin gel (except NDC^ 68682046275), clindamycin solution, clindamycin-benzoyl peroxide, erythromycin solution, erythromycin-benzoyl peroxide, tretinoin, EPIDUO, ONEXTON, TAZORAC ACIPHEX, esomeprazole, lansoprazole, omeprazole, pantoprazole, DEXILANT ACIPHEX SPRINKLE ACTICLATE doxycycline hyclate capsule, doxycycline hyclate tablet (except doxycycline hyclate tablet 50 mg [NDC^ 72143021160 only], 75 mg, 150 mg), minocycline, tetracycline ACTOS pioglitazone ACUVAIL bromfenac, diclofenac, ketorolac, PROLENSA acyclovir cream acyclovir (except acyclovir cream), valacyclovir ADCIRCA sildenafil, tadalafil ADZENYS XR-ODT amphetamine-dextroamphetamine mixed salts ext-rel†, dexmethylphenidate ext-rel, dextroamphetamine ext-rel, methylphenidate ext-rel†, MYDAYIS, -

Acitretin; Adapalene; Alitretinoin; Bexarotene; Isotretinoin
21 June 2018 EMA/261767/2018 Updated measures for pregnancy prevention during retinoid use Warning on possible risk of neuropsychiatric disorders also to be included for oral retinoids On 22 March 2018, the European Medicines Agency (EMA) completed its review of retinoid medicines, and confirmed that an update of measures for pregnancy prevention is needed. In addition, a warning on the possibility that neuropsychiatric disorders (such as depression, anxiety and mood changes) may occur will be included in the prescribing information for oral retinoids (those taken by mouth). Retinoids include the active substances acitretin, adapalene, alitretinoin, bexarotene, isotretinoin, tazarotene and tretinoin. They are taken by mouth or applied as creams or gels to treat several conditions mainly affecting the skin, including severe acne and psoriasis. Some retinoids are also used to treat certain forms of cancer. The review confirmed that oral retinoids can harm the unborn child and must not be used during pregnancy. In addition, the oral retinoids acitretin, alitretinoin and isotretinoin, which are used to treat conditions mainly affecting the skin, must be used in accordance with the conditions of a new pregnancy prevention programme by women able to have children. Topical retinoids (those applied to the skin) must also not be used during pregnancy, and by women planning to have a baby. More information is available below. Regarding the risk of neuropsychiatric disorders, the limitations of the available data did not allow to clearly establish whether this risk was due to the use of retinoids. However, considering that patients with severe skin conditions may be more vulnerable to neuropsychiatric disorders due to the nature of the disease, the prescribing information for oral retinoids will be updated to include a warning about this possible risk. -

For Health Professionals Who Care for Cancer Patients
November 2014 Volume 17, Number 11 For Health Professionals Who Care For Cancer Patients Inside This Issue: . Editor’s Choice – New Programs: nab-Paclitaxel and . Provincial Systemic Therapy Program – Reformat of Gemcitabine for Pancreatic Cancer, Neoadjuvant Paclitaxel BCCA Policy on Prevention and Management of and AC for Breast Cancer, Chlorambucil plus Rituximab for Extravasation of Chemotherapy Lymphomas, Chemotherapy plus Mitotane for . Drug Update – Enzalutamide Patient Assistance Adrenocortical Cancer Program . Cancer Drug Manual – Revised: Filgrastim, Gemcitabine, . List of New and Revised Protocols, Provincial Pre- Oxaliplatin, Paclitaxel, nab-Paclitaxel, Dexamethasone Printed Orders and Patient Handouts – New: . Medication Safety – Improving Website Clarity for Protocol BRLATACG, BRLATWAC, UGIPGEMABR, GUEDPM, Selection LYCHLRR Revised: BRAVEVEX, UCNBEV, GICIRB, . Benefit Drug List – New: BRLATACG, BRLATWAC, GIFFIRB, UGIFFOXB, UGIFIRINOX, GOCXCRT, GOENDH, UGIPGEMABR, GUEDPM, LYCHLRR Revised: ULYRMTN GUPLHRH, GUSCPE, HNAVP, LYIVACR, HNLAPRT, Deleted: BRLAACTW ULKATOATRA, ULKATOP, ULKATOR, ULUAVAFAT, LYABVD, LYCHOP, LYCHOPR, LYCHOPRMTX, LYRMTN . Continuing Professional Development – Workshop on Deleted: BRLAACTW Education in Palliative and End of Life Care for Oncology . Website Resources and Contact Information EDITOR’S CHOICE NEW PROGRAMS The Provincial Systemic Therapy Program has approved the following programs effective 1 November 2014: nab-Paclitaxel (ABRAXANE®) and Gemcitabine for Locally Advanced and Metastatic Pancreatic -
![TRETINOIN (All-Trans Retinoic Acid [ATRAC]) Oral Capsule](https://docslib.b-cdn.net/cover/0757/tretinoin-all-trans-retinoic-acid-atrac-oral-capsule-1480757.webp)
TRETINOIN (All-Trans Retinoic Acid [ATRAC]) Oral Capsule
PHARMACY COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 9/20/2018 SECTION: DRUGS LAST REVIEW DATE: 8/19/2021 LAST CRITERIA REVISION DATE: 8/19/2021 ARCHIVE DATE: TRETINOIN (all-trans retinoic acid [ATRAC]) oral capsule Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline must be read in its entirety to determine coverage eligibility, if any. This Pharmacy Coverage Guideline provides information related to coverage determinations only and does not imply that a service or treatment is clinically appropriate or inappropriate. The provider and the member are responsible for all decisions regarding the appropriateness of care. Providers should provide BCBSAZ complete medical rationale when requesting any exceptions to these guidelines. The section identified as “Description” defines or describes a service, procedure, medical device or drug and is in no way intended as a statement of medical necessity and/or coverage. The section identified as “Criteria” defines criteria to determine whether a service, procedure, medical device or drug is considered medically necessary or experimental or investigational. State or federal mandates, e.g., FEP program, may dictate that any drug, device or biological product approved by the U.S. Food and Drug Administration (FDA) may not be considered experimental or investigational and thus the drug, device or biological product may be assessed only on the basis of medical necessity. Pharmacy Coverage Guidelines are subject to change as new information becomes available. For purposes of this Pharmacy Coverage Guideline, the terms "experimental" and "investigational" are considered to be interchangeable. -

PRICE LIST – 2017 Cosmetic / Dermatology Products
PRICE LIST – 2017 Cosmetic / Dermatology Products Updated: January 2017 P: 1300 731 755 F: (07) 5530 6677 1 | P a g e Index: (A-Z) Non-PBS / Private Compounded Items ALA (Aminolevulinic Acid) Page 3 Acne Treatments Page 4 Anesthetic Products Page 5 Depigmenting Products Page 6 Hydroquinone Cream Page 6 Peel Products Page 7 Tretinoin Page 10 Jessner Peels Page 7 Peptides (Topical) Page 8 & 9 Topical Anaesthetics Page 5 Tretinoin Page 10 2 | P a g e ALA – Amino Levuleniuc Acid ** The following items require a Doctor prescription ** These are the most commonly used strengths and quantities, if it is not on the list please call us for a quote. Note: Upon arrival at your clinic please store Aminolevulinic Acid in the freezer. Instructions for use: 1. Remove Aminolevulinic Acid Hydrochloride from freezer 2. Add the Aminolevulinic Acid (5) HCI Solvent Mixture to the glass vial containing Aminolevulinic Acid Hydrochloride 3. Screw on the black phenolic cap securely and mix for one (1) – two (2) minutes with gentle shaking and swirling motion until the solid is completely dissolved. 4. Let the bottle stand for a minute or so to allow the solution to go down to the bottom of the bottle. 5. Replace the black phenolic cap with the droptainer cap and apply. Note: Use within two (2) hours of mixing and discard excess/unused solution ALA: (Strength) Quantity: Price: 1 unit $35.00 20% 2 units $49.00 3 or more units $90.00 1 unit $35.00 25% 2 units $49.00 3 or more units $90.00 3 | P a g e Acne Treatments ** The following items require a Doctor prescription ** These are the most commonly used strengths and quantities, if it is not on the list please call us for a quote. -

Bexarotene) Capsules, 75 Mg
1 PACKAGE INSERT Targretin® (bexarotene) capsules, 75 mg Rx only. Targretin® capsules are a member of the retinoid class of drugs that is associated with birth defects in humans. Targretin® capsules also caused birth defects when administered orally to pregnant rats. Targretin® capsules must not be administered to a pregnant woman. See CONTRAINDICATIONS. DESCRIPTION Targretin® (bexarotene) is a member of a subclass of retinoids that selectively activate retinoid X receptors (RXRs). These retinoid receptors have biologic activity distinct from that of retinoic acid receptors (RARs). Each soft gelatin capsule for oral administration contains 75 mg of bexarotene. The chemical name is 4-[1-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl-2- naphthalenyl) ethenyl] benzoic acid, and the structural formula is as follows: CH2 H3C CH3 CH3 COOH H3CCH3 Bexarotene is an off-white to white powder with a molecular weight of 348.48 and a molecular formula of C24H28O2. It is insoluble in water and slightly soluble in vegetable oils and ethanol, USP. Each Targretin® (bexarotene) capsule also contains the following inactive ingredients: polyethylene glycol 400, NF, polysorbate 20, NF, povidone, USP, and butylated hydroxyanisole, NF. The capsule shell contains gelatin, NF, sorbitol special-glycerin blend, and titanium dioxide, USP. CLINICAL PHARMACOLOGY Mechanism of Action Bexarotene selectively binds and activates retinoid X receptor subtypes (RXRα, RXRβ, RXRγ). RXRs can form heterodimers with various receptor partners such as retinoic acid receptors (RARs), vitamin D receptor, thyroid receptor, and peroxisome proliferator activator receptors (PPARs). Once activated, these receptors function as transcription factors that regulate the expression of genes that control cellular 2 differentiation and proliferation. -

Preferred Drug List
October 2021 Preferred Drug List The Preferred Drug List, administered by CVS Caremark® on behalf of Siemens, is a guide within select therapeutic categories for clients, plan members and health care providers. Generics should be considered the first line of prescribing. If there is no generic available, there may be more than one brand-name medicine to treat a condition. These preferred brand-name medicines are listed to help identify products that are clinically appropriate and cost-effective. Generics listed in therapeutic categories are for representational purposes only. This is not an all-inclusive list. This list represents brand products in CAPS, branded generics in upper- and lowercase Italics, and generic products in lowercase italics. PLAN MEMBER HEALTH CARE PROVIDER Your benefit plan provides you with a prescription benefit program Your patient is covered under a prescription benefit plan administered administered by CVS Caremark. Ask your doctor to consider by CVS Caremark. As a way to help manage health care costs, prescribing, when medically appropriate, a preferred medicine from authorize generic substitution whenever possible. If you believe a this list. Take this list along when you or a covered family member brand-name product is necessary, consider prescribing a brand name sees a doctor. on this list. Please note: Please note: • Your specific prescription benefit plan design may not cover • Generics should be considered the first line of prescribing. certain products or categories, regardless of their appearance in • This drug list represents a summary of prescription coverage. It is this document. Products recently approved by the U.S. Food and not all-inclusive and does not guarantee coverage. -

Cancer Drug Costs for a Month of Treatment at Initial Food and Drug
Cancer drug costs for a month of treatment at initial Food and Drug Administration approval Year of FDA Monthly Cost Monthly cost Generic name Brand name(s) approval (actual $'s) (2014 $'s) vinblastine Velban 1965 $78 $586 thioguanine, 6-TG Thioguanine Tabloid 1966 $17 $124 hydroxyurea Hydrea 1967 $14 $99 cytarabine Cytosar-U, Tarabine PFS 1969 $13 $84 procarbazine Matulane 1969 $2 $13 testolactone Teslac 1969 $179 $1,158 mitotane Lysodren 1970 $134 $816 plicamycin Mithracin 1970 $50 $305 mitomycin C Mutamycin 1974 $5 $23 dacarbazine DTIC-Dome 1975 $29 $128 lomustine CeeNU 1976 $10 $42 carmustine BiCNU, BCNU 1977 $33 $129 tamoxifen citrate Nolvadex 1977 $44 $170 cisplatin Platinol 1978 $125 $454 estramustine Emcyt 1981 $420 $1,094 streptozocin Zanosar 1982 $61 $150 etoposide, VP-16 Vepesid 1983 $181 $430 interferon alfa 2a Roferon A 1986 $742 $1,603 daunorubicin, daunomycin Cerubidine 1987 $533 $1,111 doxorubicin Adriamycin 1987 $521 $1,086 mitoxantrone Novantrone 1987 $477 $994 ifosfamide IFEX 1988 $1,667 $3,336 flutamide Eulexin 1989 $213 $406 altretamine Hexalen 1990 $341 $618 idarubicin Idamycin 1990 $227 $411 levamisole Ergamisol 1990 $105 $191 carboplatin Paraplatin 1991 $860 $1,495 fludarabine phosphate Fludara 1991 $662 $1,151 pamidronate Aredia 1991 $507 $881 pentostatin Nipent 1991 $1,767 $3,071 aldesleukin Proleukin 1992 $13,503 $22,784 melphalan Alkeran 1992 $35 $59 cladribine Leustatin, 2-CdA 1993 $764 $1,252 asparaginase Elspar 1994 $694 $1,109 paclitaxel Taxol 1994 $2,614 $4,176 pegaspargase Oncaspar 1994 $3,006 $4,802 -
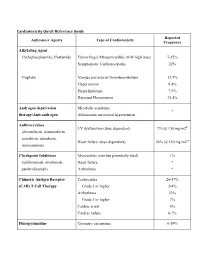
Cardiotoxicity Quick Reference Guide Reported Anticancer Agents Type of Cardiotoxicity Frequency
Cardiotoxicity Quick Reference Guide Reported Anticancer Agents Type of Cardiotoxicity Frequency Alkylating Agent Cyclophosphamide, Ifosfamide Hemorrhagic Myopericarditis (with high dose) 7-25% Symptomatic Cardiomyopathy 22% Cisplatin Venous and arterial thromboembolism 12.9% Hypertension 9.4% Hyperlipidemia 7.9% Raynaud Phenomenon 33.4% Androgen deprivation Metabolic syndrome * therapy/Anti-androgen Aldosterone associated hypertension Anthracyclines LV dysfunction (dose dependent) 7% @ 150 mg/m2Ŧ (doxorubicin, daunorubicin, epirubicin, idarubicin, Heart failure (dose dependent) 26% @ 550 mg/m2 Ŧ mitoxantrone) Checkpoint Inhibitors Myocarditis (rare but potentially fatal) 1% (ipililimumab, nivolumab, Heart failure * pembrolizumab) Arrhythmia * Chimeric Antigen Receptor Tachycardia 26-57% (CAR) T Cell Therapy Grade 3 or higher 2-4% Arrhythmia 23% Grade 3 or higher 7% Cardiac arrest 4% Cardiac failure 6-7% Fluropyrimidine Coronary vasospasm 0-19% (5-FU, capecitabine) Myocardial infarction 0-2% HER-2 targeted therapy LV dysfunction 9.4% (trastuzumab, pertuzumab) with anthracyclines 18.6% Heart failure 0.4-12% with anthracyclines 2-20% Immunomodulatory agent Venous thromboembolism 0-75% (thalidomide, lenalidomide, Arterial thromboembolism ~5% pomalidomide) Sinus bradycardia (thalidomide) 26% severe or life threatening 3% PI3K/AKT/mTOR inhibitors Hyperglycemia, hypercholesterolemia, >50% (everolimus, idelasib, hypertriglyceridemia temsirolimus) Proteasome inhibitors Hypertension 8-23% (bortezomib, carfilzomib) Heart failure (carfilzomib) 4% Radiation (mediastinal/thoracic) Arrhythmia 16% Autonomic dysfunction 30-45% Coronary heart disease 19-20% Heart failure 11-12% Pacemaker/ICD malfunction 3% Pericardial disease 5% Valvular heart disease 11-31% Tyrosine kinase inhibitors Arterial thromboembolic event (ponatinib) (see also VSP inhibitors, HER2 Atrial fibrillation (ibrutinib) antagonists and mTOR Atherosclerosis (nilotinib) inhibitors) Bleeding (ibrutinib) Incidence of adverse effects Bradyarrhythmia (trametinib) varies with specific drugs. -
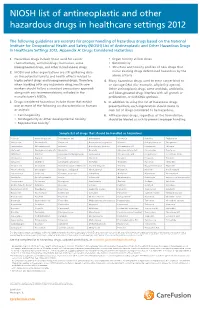
NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2012
NIOSH list of antineoplastic and other hazardous drugs in healthcare settings 2012 The following guidelines are excerpts for proper handling of hazardous drugs based on the National Institute for Occupational Health and Safety (NIOSH) List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2012, Appendix A: Drugs Considered Hazardous 1. Hazardous drugs include those used for cancer • Organ toxicity at low doses* chemotherapy, antiviral drugs, hormones, some • Genotoxicity** bioengineered drugs, and other miscellaneous drugs. • Structure and toxicity profiles of new drugs that 2. NIOSH and other organizations are still gathering data mimic existing drugs determined hazardous by the on the potential toxicity and health effects related to above criteria highly potent drugs and bioengineered drugs. Therefore, 4. Many hazardous drugs used to treat cancer bind to when working with any hazardous drug, health care or damage DNA (for example, alkylating agents). workers should follow a standard precautions approach Other antineoplastic drugs, some antivirals, antibiotics, along with any recommendations included in the and bioengineered drugs interfere with cell growth or manufacturer’s MSDSs. proliferation, or with DNA synthesis. 3. Drugs considered hazardous include those that exhibit 5. In addition to using the list of hazardous drugs one or more of the following six characteristics in humans presented here, each organization should create its or animals: own list of drugs considered to be hazardous. • Carcinogenicity 6. All hazardous