APTI415 Instructors Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Rutin on Gentamicin-Induced Ototoxicity in Rats: a Biochemical and Histopathological Examination
ORIGINAL ARTICLE ENT UPDATES 11(1):8-13 DOI: 10.5152/entupdates.2021.887158 Effect of Rutin on Gentamicin-Induced Ototoxicity in Rats: A Biochemical and Histopathological Examination Abstract Objective: Gentamicin is a broad-spectrum aminoglycoside antibiotic administered parenterally for moderate to severe gram-negative infections. Ototoxicity is an im- portant side effect that limits gentamicin use. The aim of this study is to investigate the effect of rutin on gentamicin-induced ototoxicity in rats biochemically and histo- pathologically. Methods: Distilled water was administered by oral gavage to healthy controls (HG) and cobalt administered group (GC). 50 mg/kg rutin was administered by oral gavage to rutin + gentamicin (RGG) group. After one hour, 100 mg/kg gentamicin was injected intraperitoneally (i.p) to the RGG and GC animal groups. This procedure was repeated once a day for 14 days. Results: Malondialdehyde (MDA), nuclear factor-κB(NF-κB), tumor necrosis factor alpha (TNF-α) and interleukin-1 beta(IL-1β) levels in the cochlear nerve tissue of gen- tamicin-treated animals were significantly higher compared to healthy controls and rutin + gentamicin treated rats. On the other hand, the amount of total Glutathione (tGSH) was significantly lower compared to the control and rutin group. Histopatho- logical examination revealed degenerated myelinated nerve fibers in the gentamicin group and Schwann cell nuclei were generally not seen. There was a high accumula- tion of collagen fiber in the tissue and dilated blood capillaries. In the rutin group, my- elinated nerve fibers mostly exhibited normal morphology, Schwann cell nuclei were İsmail Salcan1 evident and the vessels were normal. -

Aldrich Raman
Aldrich Raman Library Listing – 14,033 spectra This library represents the most comprehensive collection of FT-Raman spectral references available. It contains many common chemicals found in the Aldrich Handbook of Fine Chemicals. To create the Aldrich Raman Condensed Phase Library, 14,033 compounds found in the Aldrich Collection of FT-IR Spectra Edition II Library were excited with an Nd:YVO4 laser (1064 nm) using laser powers between 400 - 600 mW, measured at the sample. A Thermo FT-Raman spectrometer (with a Ge detector) was used to collect the Raman spectra. The spectra were saved in Raman Shift format. Aldrich Raman Index Compound Name Index Compound Name 4803 ((1R)-(ENDO,ANTI))-(+)-3- 4246 (+)-3-ISOPROPYL-7A- BROMOCAMPHOR-8- SULFONIC METHYLTETRAHYDRO- ACID, AMMONIUM SALT PYRROLO(2,1-B)OXAZOL-5(6H)- 2207 ((1R)-ENDO)-(+)-3- ONE, BROMOCAMPHOR, 98% 12568 (+)-4-CHOLESTEN-3-ONE, 98% 4804 ((1S)-(ENDO,ANTI))-(-)-3- 3774 (+)-5,6-O-CYCLOHEXYLIDENE-L- BROMOCAMPHOR-8- SULFONIC ASCORBIC ACID, 98% ACID, AMMONIUM SALT 11632 (+)-5-BROMO-2'-DEOXYURIDINE, 2208 ((1S)-ENDO)-(-)-3- 97% BROMOCAMPHOR, 98% 11634 (+)-5-FLUORODEOXYURIDINE, 769 ((1S)-ENDO)-(-)-BORNEOL, 99% 98+% 13454 ((2S,3S)-(+)- 11633 (+)-5-IODO-2'-DEOXYURIDINE, 98% BIS(DIPHENYLPHOSPHINO)- 4228 (+)-6-AMINOPENICILLANIC ACID, BUTANE)(N3-ALLYL)PD(II) CL04, 96% 97 8167 (+)-6-METHOXY-ALPHA-METHYL- 10297 ((3- 2- NAPHTHALENEACETIC ACID, DIMETHYLAMINO)PROPYL)TRIPH 98% ENYL- PHOSPHONIUM BROMIDE, 12586 (+)-ANDROSTA-1,4-DIENE-3,17- 99% DIONE, 98% 13458 ((R)-(+)-2,2'- 963 (+)-ARABINOGALACTAN BIS(DIPHENYLPHOSPHINO)-1,1'- -

An Overview on “Stages of Anesthesia and Some Novel General Anesthetics Drug”
Rohit Jaysing Bhor. et al. /Asian Journal of Research in Chemistry and Pharmaceutical Sciences. 5(4), 2017, 132-140. Review Article ISSN: 2349 – 7106 Asian Journal of Research in Chemistry and Pharmaceutical Sciences Journal home page: www.ajrcps.com AN OVERVIEW ON “STAGES OF ANESTHESIA AND SOME NOVEL GENERAL ANESTHETICS DRUG” Rohit Jaysing Bhor *1 , Bhadange Shubhangi 1, C. J. Bhangale 1 1* Department of Pharmaceutical Chemistry, PRES’s College of Pharmacy Chincholi, Tal-Sinner, Dist-Nasik, Maharashtra, India. ABSTRACT Anesthesia is a painless performance of medical producers. There are both major and minor risks of anesthesia. Anesthesia is a state of temporary induced loss of sensation or awareness. It gives analgesia i.e. relief from pain or prevention of pain and paralysis. General anaesthesia is a medically induced state of unconsciousness. It gives loss of protective reflux. It is carried out to allow medical procedures or medical surgery. It can be classified into 3 types like Intravenous Anesthetics Drug; Miscellaneous Drug; and Inhalational anesthetic Drug. Sodium thiopental is an ultra-short-acting barbiturate and has been used commonly in the induction phase of anesthesia. Methohexital is an example of barbiturates derivatives. It is classified as short-acting, and has a rapid onset of action. KEYWORDS Anesthesia, Sodium thiopental, Methohexital and Propofol. Author for Correspondence: INTRODUCTON Anesthesia is a state of temporary induced loss of sensation or awareness. It gives analgesia i.e. relief 1 Rohit Jaysing Bhor, from pain or prevention of pain and paralysis . A patient under the effects of anesthetic drug is known Department of Pharmaceutical Chemistry, as anesthetized. -

Reactions of Some 1-Arylpiperazines and 1-Aryl-4-(2-Hydroxy-3
Reactions of Some l-Arylpiperazines and -Aryl-4- (2-Hydroxy-3-Methoxypropyl) Piperazines By HILDA HOWELL A DISSERTATION PRESENTED TO THE GRADUATE COUNQL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA August, 1961 5 ACKNOWLEDGMENTS The author would like to thank Parke, Davis and Company who provided a graduate fellowship during part of this work. The able assistance of the faculty, staff, and fellow graduate students of the Chemistry Department has made this research quite pleasant. Thanks are also due to Miss Joan Merritt, Mrs. Marie Eckart, Mr. T. W. Brooks, and Mr. Ralph Damico for their help with the typing, drawings, and proofreading of this report. The special consideration of Mr. P. L. Bhatia who shared this laboratory is appreciated. Special recognition is offered to D». C. B. Smith, Commdr. N. L. Smith, Dr. W. M. Jones, and Dr. G. B. Butler, each of whom from his own ability and opportunity has been of inestimable help. The moral support of her parents, family, and friends has significantly contributed to the success of this graduate program. She is deeply indebted to Dr* C. B. Pollard for his inspiration and direction !n the early part of this work and to her graduate ccKnmittee for their friendship demonstrated particularly since his death. She is especially indebted to Dr. Harry H. Slsler for the under- standing way in which he has directed the completion of this research. His friexidship and encouragement have made graduate work an enjoyable experience. ^ TABLE OF CONTENTS Page ACKNOWLEDGMENTS. -

Air Pollution Control General Definitions Regulation, State Of
STATE RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF ENVIRONMENTAL MANAGEMENT OFFICE OF AIR RESOURCES AIR POLLUTION CONTROL GENERAL DEFINITIONS REGULATION Applicability Unless otherwise expressly defined by an individual Air Pollution Control Regulation, the terms, definitions and unit of measure abbreviations contained herein shall be generally applicable to all Rhode Island Air Pollution Control Regulations adopted or amended at the same time as or after the adoption of these definitions. Definitions “Act” or “Clean Air Act” or “CAA” means the Federal Clean Air Act, as amended 42 U.S.C. 7401, et seq. “Actual heat input” means the gross heat release potential based upon the actual BTU content of the fossil fuel being burned and the rate at which it is burned. “Administrator” means the Administrator of the United States Environmental Protection Agency or the Administrator's duly authorized representative. “Aerodynamic downwash” means the rapid descent of a plume to ground level with little dilution and dispersion due to alteration of background air flow characteristics caused by the presence of buildings or other obstacles in the vicinity of the emission point. “Air contaminant” means soot, cinders, ashes, any dust, fumes, gas, mist, smoke, vapor, odor, toxic or radioactive material, particulate matter, or any combination of these. “Air pollution” means the presence in the outdoor atmosphere of one or more air contaminants in sufficient quantities which, either alone or in connection with other emissions, by reason of their concentration and duration, may be injurious to human, plant or animal life, or cause damage to property or which unreasonably interferes with the enjoyment of life and property. -

Chapter 14 Ethers
Chapter 14 Ethers, Epoxides, and Sulfides Chem 233 Dr. Hoeger Introduction • Formula R-O-R′ where R and R′ are alkyl or aryl. • Symmetrical or unsymmetrical • Examples: CH3 O CH3 O O CH3 Ethers Ethers 2 1 Structure and Polarity • Bent molecular geometry • Oxygen is sp3 hybridized • Tetrahedral angle => Ethers Ethers 3 Boiling Points Similar to alkanes of comparable molecular weight. => Ethers Ethers 4 2 Hydrogen Bond Acceptor • Ethers cannot H-bond to each other. • In the presence of -OH or -NH (donor), the lone pair of electrons from ether forms a hydrogen bond with the -OH or -NH. => Ethers Ethers 5 Solvent Properties • Nonpolar solutes dissolve better in ether than in alcohol. • Ether has large dipole moment, so polar solutes also dissolve. • Ethers solvate cations. • Ethers do not react with strong bases. => Ethers Ethers 6 3 Ether Complexes • Grignard reagents H + _ • Electrophiles O B H H BH3 THF • Crown ethers => Ethers Ethers 7 Common Names of Ethers • Alkyl alkyl ether • Current rule: alphabetical order • Old rule: order of increasing complexity • Symmetrical: use dialkyl, or just alkyl. • Examples: CH3 CH3 O C CH3 CH3CH2 O CH2CH3 CH3 diethyl ether or t-butyl methyl ether or ethyl ether methyl t-butyl ether => Ethers Ethers 8 4 IUPAC Names • Alkoxy alkane • Examples: CH 3 O CH3 CH3 O C CH3 CH3 2-methyl-2-methoxypropane Methoxycyclohexane => Ethers Ethers 9 Cyclic Ethers • Heterocyclic: oxygen is in ring. O Epoxides (oxiranes) • H2C CH2 O • Oxetanes • Furans (Oxolanes ) O O • Pyrans (Oxanes ) O O O •Dioxanes => O Ethers Ethers 10 5 Naming Epoxides • Alkene oxide, from usual synthesis method H peroxybenzoic acid O cyclohexene oxide H • Epoxy attachment to parent compound, 1,2-epoxy-cyclohexane • Oxirane as parent, oxygen number 1 O H CH3 trans-2-ethyl-3-methyloxirane => CH3CH2 H Ethers Ethers 11 Spectroscopy of Ethers • IR: Compound contains oxygen, but O-H and C=O stretches are absent. -

(12) United States Patent (10) Patent No.: US 9,120,776 B2 Yamamoto Et Al
US009120776B2 (12) United States Patent (10) Patent No.: US 9,120,776 B2 Yamamoto et al. (45) Date of Patent: Sep. 1, 2015 (54) CONDENSED HETEROCYCLIC COMPOUND 2003/0171309 A1 9/2003 Halazy et al. 2003/0212012 A1 1 1/2003 Halazy et al. 2004, OO67988 A1 4/2004 Klein et al. (71) Applicant: Takeda Pharmaceutical Company 2004.0102634 A1 5/2004 Matsuura et al. Limited, Osaka-shi, Osaka (JP) 2006/0247307 A1 11/2006 Kitahara et al. 2006/0264486 A1 11/2006 Ma et al. (72) Inventors: Satoshi Yamamoto, Kanagawa (JP); 2008.0167318 A1 7/2008 Halazy et al. 2008. O176904 A1 7/2008 Govek et al. Junya Shirai, Kanagawa (JP); 2008/0221157 A1 9/2008 Chakravarty et al. Yoshiyuki Fukase, Kanagawa (JP); 2009,0163511 A1 6/2009 Darwish et al. Yoshihide Tomata, Kanagawa (JP); 2009,025.3758 A1 10, 2009 Miller Ayumu Sato, Kanagawa (JP); Atsuko 2009/0275609 A1 11/2009 Yu et al. 2009,0298854 A1 12/2009 Ma et al. Ochida, Kanagawa (JP); Kazuko 2010.0041721 A1 2, 2010 Miller Yonemori, Kanagawa (JP); Hideyuki 2011/0059958 A1 3/2011 Nishida et al. Nakagawa, Kanagawa (JP) 2011/O135650 A1 6/2011 Chackalamannil et al. 2011/0224267 A1 9, 2011 Miller (73) Assignee: Takeda Pharmaceutical Company 2011/0245222 A1 10/2011 Payan et al. Limited, Osaka (JP) 2011/0263.046 A1 10/2011 Deuschle et al. 2011/0319412 A1 12/2011 Sakagami et al. (*) Notice: Subject to any disclaimer, the term of this 2012/0108606 A1 5, 2012 Darwish et al. patent is extended or adjusted under 35 2013,0023660 A1 1/2013 Yu et al. -

Are Self-Tests (And Answers), Oa Module Assignments, and Suggested Breakpoints for Student-Teacher Consultations
DOCUMENT RESUME ED 130 858 SE 021 428 AUTHOR Torop, William TITLE Carbon..[Individualized Learning System (ILS) Chemistry Pac No. 7.] INSTITUTION West Chester State Coll., Pa. PUB DATE 76 NOTE 31p.; For related Chemistry Pads 1-8, see SE 021 422-429; Not available in hard copy due to marginal legibility of original document AVAILABLE FROMI.G.A. Bookstore, West Chester State College, West Chester, Pennsylvania 19380 ($6.95/set, plus postage) EDRS PRICE MF-$0.83 Plus Postage. HC Not Available from EDRS. DESCRIPTORS Autoinstructional kids; *Chemistry; *College Science; Computer Assisted Instruction; Higher Education; *Individualized Instruction; *Instructional Materials; *Organic Chemistry; Science Education; *Workbooks ABSTRACT This booklet is one of a set of eight designed to be used in a self-paced introductory chemistry course in conjunction with specified textbooks and computer-assisted instruction (CAI) modules. Each topic is introduced with a textbook reading assignment and additional readings are provided in the booklet. Also included are self-tests (and answers), Oa module assignments, and suggested breakpoints for student-teacher consultations. Supplementary learning materials, including filmstrips, are also suggested. Each booklet contains specific cognitive objectives to be met by completion. This booklet covers basic organic chemistry, including hydrocarbons, functional groups, and cyclic and heterocyclid-compounds. (MH) *********************************************************************** * Documents acquired by ERIC include many informal unpublished *materials not available from other sources. ERIC makes every effort* *to obtain the best copy available.Nevertheless, items of marginal * *reproducibility are often encountered and this affects the quality * *of the microfiche and hardcopy reproductions ERIC makes available * *via the ERIC Document ReproductionService (EDRS). EDRS is not *responsible for the quality of theoriginal document. -

Catalytic Dehydration and Etherification of Alcohol And
CATALYTIC DEHYDRATION AND ETHERIFICATION OF ALCOHOL AND GLYCEROL TO PRODUCE BIODIESEL COMPATIBLE FUEL ADDITIVES A Thesis by HUSAM AHMED MOHAMMED ALMASHHADANI Submitted to the Office of Graduate and Professional Studies of Texas A&M University In partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Chair of Committee, Sandun Fernando Co-Chair of Committee, Sergio Capareda Committee Member, Waruna Kulatilaka Head of Department, Stephen Searcy May 2017 Major Subject: Biological and Agricultural Engineering Copyright 2017 Husam Ahmed Mohammed Almashhadani ABSTRACT Global glycerol supplies have been increasing steadily due to the continual expansion of biodiesel production. This glut has resulted in lower demand for glycerol, price deflation and even environmental concerns. Crude glycerol produced from biodiesel transesterification is not of high quality due to catalyst and alcohol contamination and transportation and disposal issues—all of which have added further constraints to this industry. In light of this, a product that could utilize glycerol, excess alcohol and the catalyst could enhance the value proposition for the biodiesel industry. Here, we show that glycerol can be reacted with methanol and tert-butanol in the presence of common transesterification catalysts to produce an ether-rich mixture that is miscible with biodiesel. Initially, the bimolecular dehydration of two alcohols, n-propanol and methanol, with catalysts that are used in transesterification was investigated. Experiments were carried out to evaluate the feasibility of promoting the etherification reaction using methanol and n-propanol as model alcohols. When methanol and n- propanol are reacted together, three types of ethers can be produced: dimethyl ether, methyl-propyl ether (also referred to as methoxypropane), and di-propyl ether. -

United States Patent Office
3,260,760 United States Patent Office Patented July 12, 1966 2 3,260,760 chlorinated hydrocarbons to decomposition is shown by STABLIZED CHILORENATED HYDR00ARS60N the accelerated laboratory test carried out as follows; COMPOSITIONS 150 cubic centimetres of trichloroethylene, for example, Charles Domen and André Ryckaert, Brussels, Belgium, and a test piece of aluminum are placed in a 300 cubic and Charles Jegge, Zurzach, Aargau, Switzerland, as 5 signors to Solvay & Cie, Brussels, Belgium, a Belgian centimetres flask and a Soxhlet extraction apparatus pro company vided with an extractor of 65 cubic centimeters. The No Drawing. Fied (Oct. 8, 1963, Ser. No. 314,650 flask is electrically heated and the trichloroethylene is Claims priority, application Belgium, Oct. 31, 1962, rapidly brought to boiling under reflux at a constant rate 628,339 while the apparatus is traversed by a current of oxygen 6 Claims. (C. 260-652.5) 10 and illuminated by a fluorescent lamp of the “blue actnic' type. During the time of the test, the rate of release of The present invention comprises a process for the sta the acid vapours leaving the apparatus is measured. This bilization of chorinated hydrocarbons, particularly tri rate, very Small at first, becomes suddenly very large chloroethylene and perchloroethylene, with a view to while the trichloroethylene darkens in color and trans avoiding the decomposition of these products and the si 5 forms into a black tarry mass. The resistance of the tri multaneous formation of oxidation products during stor chloroethylene under test is measured by the time, ex age or during their use. -
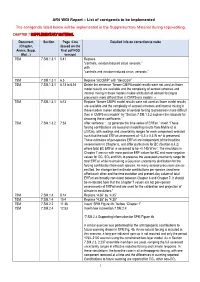
AR6 WGI Report – List of Corrigenda to Be Implemented the Corrigenda Listed Below Will Be Implemented in the Supplementary Material During Copy-Editing
AR6 WGI Report – List of corrigenda to be implemented The corrigenda listed below will be implemented in the Supplementary Material during copy-editing. CHAPTER 7 SUPPLEMENTARY MATERIAL Document Section Page :Line Detailed info on correction to make (Chapter, (based on the Annex, Supp. final pdf FGD Mat…) version) 7SM 7.SM.1.3.1 5:41 Replace “contrails, aviation induced cirrus aerosols,” with “contrails and aviation-induced cirrus, aerosols,” 7SM 7.SM.1.3.1 6.5 Replace “ACCMIP” with “AeroCom” 7SM 7.SM.1.3.1 6.13 to 6.16 Delete the sentence “Newer CMIP6 model results were not used as fewer model results are available and the complexity of aerosol schemes and internal mixing in these models makes attribution of aerosol forcing to precursors more difficult than in CMIP5-era models. » 7SM 7.SM.1.3.1 6:13 Replace “Newer CMIP6 model results were not used as fewer model results are available and the complexity of aerosol schemes and internal mixing in these models makes attribution of aerosol forcing to precursors more difficult than in CMIP5-era models” by “Section 7.SM.1.3.2 explains the rationale for choosing these coefficients.” 7SM 7.SM.1.3.2 7:53 After sentence “…to generate the time series of ERFari.” insert “These forcing contributions are based on modelling results from Myhre et al. (2013a), with scalings and uncertainty ranges for each component selected such that the total ERFari assessment of –0.3 ± 0.3 W m-2 is preserved. These estimates of per-species ERFari are independent of the headline assessments in Chapter 6, and differ particularly for BC (Section 6.4.2) where total BC ERFari is assessed to be +0.145 W m-2. -

Historical Summary of the Federal Recommended Policy on the Control of Volatile Organic Compounds and Its Implementation in Illinois
HISTORICAL SUMMARY OF THE FEDERAL RECOMMENDED POLICY ON THE CONTROL OF VOLATILE ORGANIC COMPOUNDS AND ITS IMPLEMENTATION IN ILLINOIS (Updated: November 10, 2015) Beginning in 1977, USEPA’s Recommended Policy on the Control of Volatile Organic Compounds (Recommended Policy) exempt certain chemical compounds from the definition of VOC or VOM.1 These compounds were exempt due to their negligible photochemical reactivity (i.e., their reduced capacity for partaking in the complex atmospheric chemical reactions that result in the formation in tropospheric ozone). Ultimately, in 1991, USEPA codified its Recommended Policy in the Code of Federal Regulations at 40 C.F.R. 51.100(s) in its definition of VOC. Specifically, on July 8, 1977, USEPA established its Recommended Policy in the Federal Register at 42 Fed. Reg. 35314. At that time, USEPA stated that the following compounds should be exempt from regulation due to their negligible photochemical reactivity: dichloromethane (methylene chloride) (CAS No. 75-09-2)2; ethane (CAS No. 74-84-0); methane (CAS No. 74-82-8); 1,1,1-trichloroethane (methyl chloroform) (CAS No. 71-55-6); and 1,1,2-trichloro-1,2,2-trifluoroethane (CFC-113 or Freon 113) (CAS No. 76-13-1)3. USEPA clarified its policy on June 4, 1979, at 44 Fed. Reg. 32043, and May 16, 1980, at 45 Fed. Reg. 32424. USEPA later amended its Recommended Policy by adding exempt compounds. On July 22, 1980, at 45 Fed. Reg. 48941, USEPA added five chlorofluorocarbons (CFCs) and one fluorocarbon (FC)4: 1 USEPA consistently uses the term “VOC” rather than “VOM,” but both designations refer to the same matter.