Shenandoah National Park Fisheries Monitoring Protocol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

People, Land, and Water of the Upper Thornton River Watershed: a Model for Countywide Watershed Management Planning
People, land, and water of the Upper Thornton River Watershed: A model for countywide watershed management planning Final Report to the National Fish and Wildlife Foundation RappFLOW Grant #2005-0001-039 Prepared by Beverly Hunter, Project Director [email protected] Phone: 540 937-4038 This report is available in pdf format from www.rappflow.org November 29, 2006 12/18/2006 1 Introduction Rappahannock Friends and Lovers of Our Watershed (RappFLOW) is a group of volunteers founded in the summer of 2002. We work with many partner organizations as well as local leaders, landowners, and other stakeholders to help preserve, protect and restore the watersheds and water quality in Rappahanock County, Virginia. In this report, we provide highlights of the results, lessons learned and progress made from March 2005 through November 2006 in the project “People, land, and water of the Upper Thornton River Watershed: A model for countywide watershed management planning.” This project was funded in part by the National Fish and Wildlife Foundation, the Virginia Department of Conservation and Recreation, the County of Rappahannock, nonprofit organizations, and private donors. Several state agencies provided technical assistance and training. Rappahannock County is a rural, scenic county with a population of about 7,000 at the headwaters of the Rappahannock River Basin. Seven hundred and fifty-five (755) stream miles in 1,010 stream segments (National Hydrology Database 2005), many on steep slopes, crisscross our land area of about 267 square miles. The northwestern boundary is in the Blue Ridge Mountains, in Shenandoah National Park. The Rappahannock River forms the northeastern boundary with Fauquier County. -

Most Effective Basins Funding Allocations Rationale May 18, 2020
Most Effective Basins Funding Allocations Rationale May 18, 2020 U.S. Environmental Protection Agency Chesapeake Bay Program Office Most Effective Basins Funding In the U.S. Environmental Protection Agency’s (EPA) Fiscal Year (FY) 2020 Appropriations Conference Report, an increase to the Chesapeake Bay Program (CBP) Budget was provided in the amount of $6 million for “state-based implementation in the most effective basins.” This document describes the methodology EPA followed to establish the most effective use of these funds and the best locations for these practices to be implemented to make the greatest progress toward achieving water quality standards in the Chesapeake Bay. The most effective basins to reduce the effects of excess nutrient loading to the Bay were determined considering two factors: cost effectiveness and load effectiveness. Cost effectiveness was considered as a factor to assure these additional funds result in state-based implementation of practices that achieve the greatest benefit to water quality overall. It was evaluated by looking at what the jurisdictions have reported in their Phase III Watershed Implementation Plans (WIPs) as the focus of their upcoming efforts, and by looking at the average cost per pound of reduction for BMP implementation by sector. Past analyses of cost per pound of reduction have shown that reducing nitrogen is less costly by far than reducing phosphorus1. Based on that fact, EPA determined that the focus of this evaluation would be to target nitrogen reductions in the watershed. Evaluating the load reduction targets in all the jurisdictions’ Phase III WIPs shows that the agricultural sector is targeted for 86 percent of the overall reductions identified to meet the 2025 targets collectively set by the jurisdictions. -
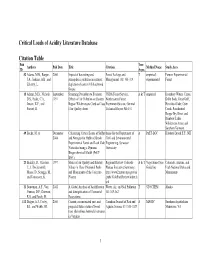
Critical Loads of Acidity Database Tables
Critical Loads of Acidity Literature Database Citation Table Pub Terr. Authors Pub Date Title Citation Method Name Study Area ID Aqua. 52 Adams, M.B., Burger, 2000 Impact of harvesting and Forest Ecology and T empirical: Fernow Experimental J.A., Jenkins, A.B., and atmospheric pollution on nutrient Management 138: 301-319 experimental Forest Zelazny, L. depletion of eastern US hardwood forests. 15 Adams, M.B., Nichols, September Screening Procedure to Evaluate USDA Forest Service, A & T empirical Boundary Waters Canoe, D.S., Feder, C.A., 1991 Effects of Air Pollution on Eastern Northeastern Forest Dolly Sods, Great Gulf, Jensen, K.F., and Region Wildernesses Cited as Class Experiment Station; General Hercules-Glade, Otter Parrott, H. I Air Quality Areas. Technical Report NE-151 Creek, Presidential Range-Dry River, and Rainbow Lakes Wilderness Areas, and Southern Vermont 49 Backx, M. A. December Calculating Critical Loads of Sulfur thesis for the Department of A PnET-BGC Hubbard Brook E.F., NH 2004 and Nitrogen for Hubbard Brook Civil and Environmental Experimental Forest and Loch Vale Engineering, Syracuse Watershed using a Dynamic University Biogeochemical Model (PnET- BGC). 21 Binkley, D., Giardina, 1997 Status of Air Quality and Related Regional Review Colorado A & T Vegetation-Type Colorado, Arizona, and C., I. Dockersmith, Values in Class I National Parks Plateau Executive Summary: Guideline Utah National Parks and Morse, D., Scruggs, M., and Monuments of the Colorado http://www2.nature.nps.gov/air Monuments and Tonnessen, K. Plateau /pubs/ColoPlat.Review/index.h tml 23 Bouwman, A.F., Van 2002 A Global Analysis of Acidification Water, Air, and Soil Pollution T STOCHEM Alaska Vuuren, D.P., Derwent, and Eutrophication of Terrestrial 141:349-382 R.G. -

Storm Data and Unusual Weather Phenomena - December 2018
Storm Data and Unusual Weather Phenomena - December 2018 Location Date/Time Deaths & Property & Event Type and Details Injuries Crop Dmg DISTRICT OF COLUMBIA DISTRICT OF COLUMBIA COUNTY --- 1.3 SW WALTER REED [38.97, -77.05], 0.7 SW WALTER REED [38.97, -77.04], 0.5 WSW WALTER REED [38.98, -77.04], 0.6 WSW WALTER REED [38.98, -77.04], 1.0 SW WALTER REED [38.97, -77.05], 1.4 SW WALTER REED [38.97, -77.05] 12/15/18 20:25 EST 0 Flood (due to Heavy Rain) 12/16/18 07:30 EST 0 Source: River/Stream Gage The stream gage on Rock Creek at Sherrill Drive/DC exceeded the 7 foot flood stage during the indicated period. Water reached several portions of the Valley Trail between Picnic Areas 7 and 10 in Rock Creek Park, and completely covered the Rolling Meadows Trailbridge. The peak level of 8.02 feet occurred at 3:45 AM on the 16th. A potent upper-level low and associated area of surface low pressure approached the region from southwest on the 15th. Strong forcing for ascent and ample moisture transport in advance of the upper level low led to a prolonged period of rain across the region. Eventually low pressure transferred to the coast before precipitation wound down on the 16th. Widespread rainfall totals of 2-4 inches were observed across the area. (DC-Z001) DISTRICT OF COLUMBIA 12/17/18 00:47 EST 0 Coastal Flood 12/18/18 08:30 EST 0 An influx of freshwater due to heavy rain led to increased water level anomalies and coastal flooding along the Potomac River. -
Easygrants ID: 18695 NFWF/Legacy Grant Project ID: 0603.09.018695
Easygrants ID: 18695 National Fish and Wildlife Foundation NFWF/Legacy Grant Project ID: 0603.09.018695 Chesapeake Bay Small Watershed Grants 2009 - Submit Final Programmatic Report (Activities) Grantee Organization: Trout Unlimited, Inc. Project Title: Thornton River Dam Removal (VA) Project Period 09/01/2009 - 12/31/2010 Award Amount $72,933.00 Matching Contributions $18,460.00 Project Location Description (from Proposal) The project site is approximately 1 km downstream of the confluence of the North Fork and South Fork of the Thornton River on the main stem Thornton River, in Sperryville, VA. Project Summary (from Proposal) Removed an aging four foot high, 80 foot long dam on the Thornton River that blocks fish migration. Project allowed four species of diadromous fish that formerly migrated into the Rappahannock watershed to access 93 kilometers of high quality upstream habitat. Summary of Accomplishments Trout Unlimited has met all objectives stated for the Thornton River Dam Removal Project. In December 2009, the dam was removed from the Thornton River by contractors using heavy equipment. The old dam material was buried offsite in the floodplain and incorporated into the two constructed floodplains that were made. Two large rock vanes using quarry stone were constructed as designed spanning the river channel and both active floodplains. Woody debris was incorporated into the rock vanes, which were tied into the floodplain elevation. The old mill raceway located between the dam and the old Fletcher’s Mill was blocked with large rock and material to eliminate the threat of a channel avulsion. A constructed wetland was created on the east margin on the upstream constructed floodplain. -

Upper Hazel River TMDL Implementation Plan 1
Upper Hazel River Bacteria Total Maximum Daily Load Plan Technical Report Submitted to: Virginia Department of Conservation & Recreation and Virginia Department of Environmental Quality Prepared by: Engineering Concepts, Inc. Submitted: June 2009 TABLE OF CONTENTS TABLES ..........................................................................................................................................3 FIGURES.........................................................................................................................................3 ACKNOWLEDGEMENTS.............................................................................................................4 1. EXECUTIVE SUMMARY.....................................................................................................5 Introduction................................................................................................................................. 5 Review of TMDL Study ............................................................................................................. 6 Public Participation..................................................................................................................... 6 Implementation Actions.............................................................................................................. 7 Measurable Goals and Milestones for Attaining Water Quality Standards................................ 8 Stakeholder’s Roles and Responsibilities .................................................................................. -

Hazel River Success Story
Implementing Best Management Practices and Other Restoration Efforts to Improve the Quality of Streams in the Upper Hazel Watershed Impaired Waterbody Improved: The Hazel River watershed covers approximately 225,990 acres and includes the Hughes, Rush, Thornton and Hazel Rivers (Figure 1). The Hazel River originates in Rappahannock County and continues downstream to its confluence with the Rappahannock River. The Virginia Department of Environmental Quality (DEQ) first listed the Hazel River and its tributaries on Virginia’s 303(d) list of impaired waters for violations of the bacteria water quality standard in 2002 and 2004. A Total Maximum Daily Load (TMDL) study was completed to address these impairments in 2007. In June 2009, a TMDL Implementation Plan (IP) was completed, followed by a 319 grant funded implementation project that began in July 2009. Additionally, this project has been awarded a Section 319(h) funds for implementation through June 2019. To reduce bacteria loadings, various agricultural and residential best management practices (BMPs) have been employed; through a mix of 319(h) and other federal, state, landowner, and private foundation funds and incentives. Implementing agricultural and septic system BMPS on the ground and providing sewer service to approximately 98 households/businesses in the Rush River watershed has reduced bacterial inputs both from point and non-point sources. Stream quality is beginning to respond to these efforts, as can be seen in Figure 2 and Figure 3. Figure 1. Map of the Upper Hazel River TMDL IP Watershed. Cleaning up the Hazel: The Actions of Many Even before the IP was developed, diverse partnerships formed to address the impaired streams in the area. -

WSP for Rappahannock County and Washington
The Water Supply Plan for Rappahannock County and the Town of Washington Prepared By: Tim Bondelid Consulting Engineer and Systems Analyst September 2011 1 Table of Contents List of Figures..................................................................................................................... 5 List of Tables ...................................................................................................................... 6 Executive Summary............................................................................................................ 7 Objective......................................................................................................................... 7 Summary of Findings ..................................................................................................... 7 List of Acronyms ................................................................................................................ 9 SECTION I: Overview of Rappahannock County ........................................................... 10 Physical Description ..................................................................................................... 10 Population..................................................................................................................... 13 Residential Water Usage............................................................................................... 13 Climate......................................................................................................................... -

Rappahannock Watershed
Rappahannock Watershed County Culpeper Stream DEQ Station ID Latitude Location Date Hydrologic method: Longitude Monitored Unit Code Score Condition Cedar Run 3CED-C2-SOS 38 21 48 Under 522 bridge 7/19/2001 E16 modified 77 58 33 8 Acceptable Cedar Run 3CED-C2-SOS 38 21 48 Under 522 bridge 1/28/2002 E16 modified 77 58 33 11 Acceptable Cedar Run 3CED-C2-SOS 38 21 48 Under 522 bridge 3/28/2001 E16 modified 77 58 33 5 Unacceptable Cedar Run 3CED-C2-SOS 38 21 48 Under 522 bridge 10/26/2001 E16 modified 77 58 33 10 Acceptable Cedar Run 3CED-C2-SOS 38 21 48 Under 522 bridge 4/26/2002 E16 modified 77 58 33 9 Acceptable Crooked Run 3COO-C1-SOS 38 21 13 100' upst from Rt 15 bridge 10/26/2001 E15 modified 78 06 49 10 Acceptable Crooked Run 3COO-C1-SOS 38 21 13 100' upst from Rt 15 bridge 8/5/2001 E15 modified 78 06 49 8 Acceptable Crooked Run 3COO-C1-SOS 38 21 13 100' upst from Rt 15 bridge 4/11/2002 E15 modified 78 06 49 11 Acceptable Crooked Run 3COO-C1-SOS 38 21 13 100' upst from Rt 15 bridge 3/28/2001 E15 modified 78 06 49 5 Unacceptable Page 1 of 20 Crooked Run 3COO-C1-SOS 38 21 13 100' upst from Rt 15 bridge 2/6/2002 E15 modified 78 06 49 9 Acceptable Hazel River 3HAZ-C5-SOS 38 31 35 Rt 644 8/8/2001 E04 modified 78 10 20 10 Acceptable Hazel River 3HAZ-C5-SOS 38 31 35 Rt 644 4/26/2002 E04 modified 78 10 20 9 Acceptable Hazel River 3HAZ-C5-SOS 38 31 35 Rt 644 10/23/2001 E04 modified 78 10 20 9 Acceptable Hazel River 3HAZ-C5-SOS 38 31 35 Rt 644 1/11/2002 E04 modified 78 10 20 7 Acceptable Hazel River 3HAZ-C5-SOS 38 31 35 Rt 644 5/3/2001 E04 modified -

Jordan's Point Dam Removal
Chapter Officers: Fall Edition: September 2019 Jordan’s Point Dam Removal President: Brad Fink In collaboration with its project partners, the City of Lexington and the U.S. Fish and Wildlife Ser- [email protected] vice, the Department of Game and Inland Fisheries recently completed the partial removal of Jordan's Point Dam (pictured below) on the Maury River in Lexington, Virginia. After years of planning and months of waiting for the river to reach acceptably low flows, the compromised President:-Elect: structure has successfully been removed. The Maury River now flows freely through the previ- Vic DiCenzo ously impounded 1.2-mile reach, including Jordan’s Point Park. [email protected] Improved public safety, restoration to riverine habitat and geomorphology, and removal of a fish barrier were the goals of this project. The Maury River supports a wide diversity of fish life, alt- Immediate Past-President: hough diadromous species are conspicuously absent from its waters. Important native species Johnathan Harris [email protected] such as Bluehead Chub, River Chub, Fallfish, White Sucker, Torrent Sucker, Longfin Darter and Redbreast Sunfish will benefit from a -re connected river. Radio-tagged feral Smallmouth Bass, a naturalized species to the Maury River, have been followed by students from Washington and Secretary: Lee University before the dam was removed. This study will continue for at least another year or Kirk Smith more. [email protected] Historically, the Maury River has supported sensitive mussel fauna such as the James Spinymus- Treasurer: sel, Dwarf Wedgemussel, and Green Floater. These three species have largely been extirpated Karen Horodysky from the Maury River. -

REPORT NUMBER: SEPTEMBER 1, 2017 – M ARCH 31, 2018 F The
GRANT TITLE: PROMOTING STRATEGIC FISH HABITAT CONSERVATION THROUGH REGIONAL AND COLLABORATIVE SCIENCE AND PRIORITY SETTING REPORT NUMBER: SEPTEMBER 1, 2017 – MARCH 31, 2018 FINAL REPORT The objective of this portion of the 2017 Multistate Conservation Grant to Fish Habitat Partnerships was to have the Atlantic Coastal Fish Habitat Partnership (ACFHP), Southeast Aquatic Resources Partnership (SARP), and Eastern Brook Trout Joint Venture (EBTJV) collectively define and communicate the scientific basis of river restoration through connectivity improvement throughout the Eastern U.S. The specific milestones outlined to address this objective included: • Finalize a tiered listing of prioritized fish habitat connectivity focus areas. • Coordinate with partners to identify and prioritize watersheds for conservation where fragmentation of, or barriers to, fish passage are a potentially critical threat to be addressed. • Develop a ‘standardized tool box’ of fish passage technologies and guidance to assist the public in development and implementation of effective fish passage protocols. Accomplishments to Meet the Objectives ACFHP finalized their priority drainages (HUC8) from the original list developed in 2016 (original list: Appendix I, ACFHP’s priority list: Appendix II). These priorities were based on the number of diadromous fish species present in each drainage. Alewife, blueback herring, and American shad presence data came from the alosine assessment in the Fish Habitat Decision Support Tool (www.fishhabitattool.org), which was developed by ACFHP, Downstream Strategies, The Nature Conservancy, and the North Atlantic Landscape Conservation Cooperative. Atlantic sturgeon data was based on Atlantic sturgeon Critical Habitat designations, and American eel presence was determined by expert opinion. The priority drainages were confirmed to have at least three species present, whereas the other potential drainages had only one confirmed species present or less. -

Ordinance of Chapter 7, Elections
BOARD OF SUPERVISORS OF CULPEPER COUNTY, VIRGINIA ORDINANCE AMENDING CHAPTER 7 (ELECTIONS ) OF THE CODE OF THE COUNTY OF CULPEPER, VIRGINIA WHEREAS, 15.2—1427 and 15.2-1433 of the Code of Virginia, 1950, as may be amended from time to time, enable a local governing body to adopt, amend and codify ordinances or portions thereof; and WHEREAS, the proper advertisement and public hearings were conducted as required by law; and WHEREAS, the full text of this amendment provided below was available for public review in the Culpeper County Administrators Office, 302 N. Main Street, Culpeper, Virginia 22701; and in the Circuit Court Clerks Office at the Culpeper County Courthouse, and NOW, THEREFORE, BE IT ORDAINED that the Board of Supervisors of Culpeper County hereby amends Chapter 7 (ELECTIONS) of the Code of the County of Culpeper, Virginia as follows: Summarized Below, This amendment seeks to redraw magisterial districts so as to give, as nearly as practicable, representation in proportion to the population in the district (ie:, one person — one vote). This effort is constitutionally required every ten years, and is to be done utilizing newly released census figures. The effect of the proposed Ordinance would be to slightly increase the population size of the Jefferson, Stevensburg and Salem Districts. The Catalpa and Cedar Mountain Districts would be significantly increased, and both would include areas within the Town Corporate Limits. The East and West Fairfax Districts would both be reduced in population size. Two new precincts would also be created, one in Catalpa and one in Cedar Mountain. AND, BE IT FURTHER RESOLVED that the Board of Supervisors of Culpeper County hereby adopts and incorporates by reference the attached magisterial district and precinct boundary maps.