WO 2017/178645 Al 19 October 2017 (19.10.2017) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessment of Bone Conduction Thresholds After Surgical Treatment in Patients with Labyrinthine Fistula
Turkish Archives of Otorhinolaryngology Turk Arch Otorhinolaryngol 2018; 56(2): 89-94 89 Türk Otorinolarengoloji Arşivi Assessment of Bone Conduction Thresholds After Surgical Treatment in Patients with Labyrinthine Fistula Müzeyyen Yıldırım Baylan1 , Ümit Yılmaz1 , Zeki Akkuş2 , İsmail Topçu1 1Department of Otorhinolaryngology, Dicle University School of Medicine, Diyarbakır, Turkey Original Investigation 2Department of Biostatistics, Dicle University School of Medicine, Diyarbakır, Turkey Abstract Objective: This study aimed to analyze the bone con- years. In the post-operative period, it was possib- duction thresholds before and after surgery in chronic le to conduct audiological follow-up on 20 patients. otitis media patients with cholesteatoma who had In these follow-ups, 16 patients showed no change labyrinthine fistula and whose cholesteatoma matrix in bone conduction thresholds, two patients showed had been completely cleaned. worsening, and two showed improvement. When Methods: The study was performed between 2013 pre- and post-operative bone conduction thresholds to 2017 with 23 chronic otitis media patients who at each frequency were compared separately, no sig- had labyrinthine fistula with cholesteatoma and who were operated at the Department of Otorhinolar- nificant difference was found (p=0.937). No statis- yngology of Dicle University School of Medicine. tically significant difference was found between the Patients were assessed by anamnesis and examina- pre- and post-operative means at the four frequencies tion and when necessary, by temporal computerized (p=0.712). tomography and diffusion magnetic resonance ima- Conclusion: In this study, we found that to reduce ging. Bone conduction thresholds at frequencies of complications relating to cholesteatoma, it might be 500, 1000, 2000, and 4000 Hz were determined by audiometric examination and they were compared necessary to completely remove the matrix especially before and after surgery. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

A Randomized Double-Blind, Double-Dummy
pISSN 1598-2998, eISSN 2005-9256 Cancer Res Treat. 2014;46(1):19-26 http://dx.doi.org/10.4143/crt.2014.46.1.19 Original Article Open Access A Randomized Double-Blind, Double-Dummy, Multicenter Trial of Azasetron versus Ondansetron to Evaluate Efficacy and Safety in the Prevention of Delayed Nausea and Vomiting Induced by Chemotherapy Hee Yeon Lee, MD 1 Purpose Hoon-Kyo Kim, MD 1 This study was conducted to evaluate the efficacy and safety of azasetron compared Kyung Hee Lee, MD 2 to ondansetron in the prevention of delayed chemotherapy-induced nausea and Bong-Seog Kim, MD 3 vomiting. MD 4 Hong Suk Song, Materials and Methods MD 5 Sung Hyun Yang, This study was a multi-center, prospective, randomized, double-dummy, double-blind MD 6 Joon Hee Kim, and parallel-group trial involving 12 institutions in Korea between May 2005 and MD 7 Yeul Hong Kim, December 2005. A total of 265 patients with moderately and highly emetogenic MD 8 Jong Gwang Kim, chemotherapy were included and randomly assigned to either the azasetron or MD 9 Sang-We Kim, ondansetron group. All patients received azasetron (10 mg intravenously) and MD 10 Dong-Wan Kim, dexamethasone (20 mg intravenously) on day 1 and dexamethasone (4 mg orally every MD 11 Si-Young Kim, 12 hours) on days 2-4. The azasetron group received azasetron (10 mg orally) with MD 12 Hee Sook Park, placebo of ondansetron (orally every 12 hours), and the ondansetron group received Department of Internal Medicine, ondansetron (8 mg orally every 12 hours) with placebo of azasetron (orally) on days 1St. -
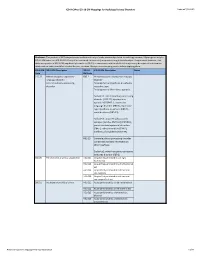
ICD-9/10 Mapping Spreadsheet
ICD-9-CM to ICD-10-CM Mappings for Audiology Related Disorders Updated 7/16/2015 Disclaimer: This product is NOT comprehensive and consists only of codes commonly related to audiology services. Mappings are only to ICD-10-CM codes, not ICD-10-PCS. Every effort was made to accurately map codes using detailed analysis. Keep in mind, however, that while many codes in ICD-9-CM map directly to codes in ICD-10, in some cases, additional clinical analysis may be required to determine which code or codes should be selected for your situation. Always review mapping results before applying them. ICD-9-CM ICD-9-CM Description ICD-10- ICD-10-CM Description Notes Code CM Code 315.32 Mixed receptive-expressive F80.2 Mixed receptive-expressive language language disorder disorder Central auditory processing Developmental dysphasia or aphasia, disorder receptive type Developmental Wernicke's aphasia Excludes1: central auditory processing disorder (H93.25), dysphasia or aphasia NOS (R47.-), expressive language disorder (F80.1), expressive type dysphasia or aphasia (F80.1), word deafness (H93.25) Excludes2: acquired aphasia with epilepsy [Landau-Kleffner] (G40.80-), pervasive developmental disorders (F84.-), selective mutism (F94.0), intellectual disabilities (F70-F79) H93.25 Central auditory processing disorder Congenital auditory imperception Word deafness Excludes1: mixed receptive-epxressive language disorder (F80.2) 380.00 Perichondritis of pinna, unspecified H61.001 Unspecified perichondritis of right external ear H61.002 Unspecified perichondritis -

Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond
Published August 25, 2016 as 10.3174/ajnr.A4922 REVIEW ARTICLE Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond X M.-L. Ho, X G. Moonis, X C.F. Halpin, and X H.D. Curtin ABSTRACT SUMMARY: Third window abnormalities are defects in the integrity of the bony structure of the inner ear, classically producing sound-/ pressure-induced vertigo (Tullio and Hennebert signs) and/or a low-frequency air-bone gap by audiometry. Specific anatomic defects include semicircular canal dehiscence, perilabyrinthine fistula, enlarged vestibular aqueduct, dehiscence of the scala vestibuli side of the cochlea, X-linked stapes gusher, and bone dyscrasias. We discuss these various entities and provide key examples from our institutional teaching file with a discussion of symptomatology, temporal bone CT, audiometry, and vestibular-evoked myogenic potentials. ABBREVIATIONS: EVAS ϭ enlarged vestibular aqueduct syndrome; SSCCD ϭ superior semicircular canal dehiscence hird window abnormalities are defects in the integrity of the ing effects decrease air conduction. The 2 physiologic windows Tbony structure of the inner ear, first described by Minor et al between the middle and inner ear are the oval window, which in 1998.1 In 2008, Merchant and Rosowski2 proposed a universal transmits vibrations from the auditory ossicles, and the round theory for the underlying mechanism of hearing loss accompany- window of the cochlea. With air conduction, there is physiologic ing these defects. Normal sound conduction is transmitted entrainment of the oval and round windows due to coupling by through the oval and round windows, which serve as fluid inter- incompressible perilymph. Pressure differences between the co- faces between air in the middle ear and perilymphatic fluid spaces chlear perilymphatic spaces activate hair cells and create the per- of the inner ear. -

Download Product Insert (PDF)
PRODUCT INFORMATION Azasetron (hydrochloride) Item No. 28383 CAS Registry No.: 123040-16-4 Formal Name: N-1-azabicyclo[2.2.2]oct-3-yl-6-chloro-3,4- dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine- Cl N 8-carboxamide, monohydrochloride O Synonym: Y-25130 MF: C H ClN O • HCl N 17 20 3 3 O FW: 386.3 Purity: ≥98% • HCl N O UV/Vis.: λmax: 221, 307 nm Supplied as: A crystalline solid H Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Description Azasetron is an orally bioavailable antagonist of the serotonin (5-HT) receptor subtype 5-HT3 1,2 (Ki = 2.9 nM). It is selective for 5-HT3 over 5-HT1A and 5-HT2 (IC50 = >10 μM for both), as well as dopamine D1 and D2 and α1- and α2-adrenergic receptors (IC50s = >10 μM), but it does bind to histamine H1 receptors (IC50 = 4.4 μM). Azasetron inhibits contractions induced by 5-HT (Item No. 14332) in isolated guinea pig ileum (pA2 = 7.04) and isolated rabbit heart (pA2 = 10.06). It inhibits emesis induced by cisplatin (Item No. 13119) in dogs and induced by doxorubicin (Item No. 15007) combined with cyclophosphamide (Item No. 13849) in ferrets when administered at a dose of 1 mg/kg.1 References 1. Haga, K., Inaba, K., Shoji, H., et al. The effects of orally administered Y-25130, a selective serotonin3-receptor antagonist, on chemotherapeutic agent-induced emesis. Jpn. J. Pharmacol. 63(3), 377-383 (1993). -

Simultaneous Determination of Dexamethasone, Ondansetron, Granisetron, Tropisetron, and Azasetron in Infusion Samples by HPLC with DAD Detection
Hindawi Publishing Corporation Journal of Analytical Methods in Chemistry Volume 2017, Article ID 6749087, 7 pages http://dx.doi.org/10.1155/2017/6749087 Research Article Simultaneous Determination of Dexamethasone, Ondansetron, Granisetron, Tropisetron, and Azasetron in Infusion Samples by HPLC with DAD Detection Fu-chao Chen,1 Lin-hai Wang,2 Jun Guo,3 Xiao-ya Shi,1 and Bao-xia Fang1 1 Department of Pharmacy, Dongfeng Hospital, Hubei University of Medicine, Shiyan, Hubei 442008, China 2Department of Pharmacy, Renmin Hospital, Hubei University of Medicine, Shiyan, Hubei 442000, China 3Department of Oncology, Dongfeng Hospital, Hubei University of Medicine, Shiyan, Hubei 442008, China Correspondence should be addressed to Bao-xia Fang; [email protected] Received 21 October 2016; Accepted 19 December 2016; Published 11 January 2017 Academic Editor: Chih-Ching Huang Copyright © 2017 Fu-chao Chen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A simple and rapid high-performance liquid chromatography with diode array detector (HPLC-DAD) method has been developed and validated for simultaneous quantification of five antiemetic agents in infusion samples: dexamethasone, ondansetron, granisetron, tropisetron, and azasetron. The chromatographic separation was achieved on a Phenomenex18 C column (4.6 mm × 150 mm, 5 m) using acetonitrile-50 mM KH2PO4 buffer-triethylamine (25 : 74 : 1; v/v; pH 4.0). Flow rate was 1.0 mL/min witha ∘ column temperature of 30 C. Validation of the method was made in terms of specificity, linearity, accuracy, and intra- and interday precision, as well as quantification and detection limits. -

Journal of Addiction and Dependence
Journal of Addiction and Dependence www.ommegaonline.org Review Article Molecular Mechanisms of Drug Abuse, Dependency and Craving Richard E. Wilcox1*, Joseph D. Miller2 1Division of Pharmacology and Toxicology, College of Pharmacy, The University of Texas at Austin, Austin TX 78712, USA 2Department of Pharmacology, American University of the Caribbean School of Medicine, Cupecoy, St. Maarten *Corresponding author: Richard E. Wilcox, Ph.D, Professor of Neuropharmacology, University of Texas, College of Pharmacy 2409, University Avenue STOP A1900 Austin, TX 78712-1113; Tel: +(512) 471-5199; E-mail: [email protected] Abstract Received Date: November 4, 2015 Drug abuse and dependence are major medical, social, and economic prob- Accepted Date: December 30, 2015 lems for the world. Whereas the means to reduce abuse are well known, drug de- Published Date: January 5, 2016 pendency is a complex medical disease and current treatments attempt to reduce or prevent drug craving in dependent people as part of therapy. With the increased under- Citation: Wilcox, R.E., et al. Molecular standing of the neural mechanisms of drug dependence and the availability of several Mechanisms of Drug Abuse, Dependen- drugs that can treat craving in certain drug dependent populations it is important to cy and Craving. (2016) J Addict Depend summarize development of anti-craving therapeutics world-wide. The present paper 2(1): 40- 51. briefly outlines the problems of drug abuse and dependence, key aspects of the drug dependence process, the nature and mechanisms of drug craving in dependent people, current drug dependence theories, and finally mechanisms of action of anti-craving agents. -

The Coexistence of Labyrinthine Fistula and the Facial Canal Dehiscence
The Mediterranean Journal of Otology ORIGINAL ARTICLE Management of Labyrinthine Fistula and Accompanying Findings: The Coexistence of Labyrinthine Fistula and the Facial Canal Dehiscence Masoud Naderpour, Ghodrat Mohammadi, Najmeh Doostmohammadian Department of Otorhinolaryngology, Tabriz University of Medical science, Tabriz, Iran OBJECTIVE: To describe the audio-vestibular results of labyrinthine fistula surgery in patients with cholesteatoma. Correspondent Author: PATIENTS AND METHODS: Data of 185 patients who had undergone Chodrat Mohammadi Dept. Otorhnotaryngology Tabriz surgery for cholesteatoma between 2001 and 2007 were reviewed. University of Medical Sciences, Three-layer sealing was used for the management of fistula. Tabriz, ‹ran RESULTS: Twenty patients were found to have labyrinthine fistula, of which 11 (55%) were male and 9(45%) female. Fistula wase located in lateral Tel: + 98- 9141141619 semicircular canal in all cases. Correlation of labyrinthine fistula and facial E-mail: [email protected] nerve dehiscence was statistically significant. Follow up was done for 1- 6 year. Postoperatively, vertigo disappeared in 19 (95 %) patients. Submitted: 14 April 2008 Revised: 10 July 2008 Hearing remained unchanged in 18 (90 %) patients. Worsening in bone Accepted: 17 July 2008 conduction thresholds was observed in 2 (10 %) patients. Postoperative deafness did not occur. Mediterr J Otol 2008; 4: 132-137 CONCLUSION: Possibility of facial nerve dehiscence and tegmen defect should be considered in patients with labyrinthine fistula. Three-layer Copyright 2005 © The Mediterranean sealing may be a valuable technique in surgical treatment of labyrinthine Society of Otology and Audiology fistula, lowering the risk of cochleovestibular functions. 132 Management of labyrinthine fistula and accompanying findings Cholesteatoma is a pocket or cystic lesion consisting of Labyrinthine fistula (LF) is encountered during stratified squamous epithelium and proliferative keratin surgery for cholesteatoma with an average frequency within the temporal bone. -

Positive Perilymph Fistula Test with Semicircular Canal Dehiscence from Cholesteatoma
PRACTICE | CLINICAL IMAGES Positive perilymph fistula test with semicircular canal dehiscence from cholesteatoma Ming-Chih Hsieh MD, Chen Chi Wu MD PhD, Shih-Hao Wang MD n Cite as: CMAJ 2019 January 28;191:E104. doi: 10.1503/cmaj.180799 54-year-old man presented to our outpatient department with left-side hearing loss and tinnitus that had progressed for several years. The patient had vertigo with nausea, whichA was aggravated on applying pressure over the left external ear canal and tragus. Physical examination showed left-side tym- panic membrane retraction with a whitish mass at the epitympa- num, suggestive of cholesteatoma. Gently compressing the left-ear tragus induced apparently left-beating horizontal nystagmus (see video, Appendix 1, available at www.cmaj.ca/lookup/suppl/ doi:10.1503/cmaj.180799/-/DC1), consistent with a positive peri- lymph fistula test. Pure tone audiometry showed mixed-type hear- ing loss of 104 dB in the patient’s left ear and sensorineural hearing loss of 62 dB in his right. High-resolution computed tomography (CT) scan of the patient’s temporal bone showed a soft-tissue mass in his left middle ear and mastoid cavity with left lateral semicircular canal erosion (Appendix 2, available at www.cmaj.ca/lookup/suppl/ Figure 1: Microscopic view of left lateral semicircular canal dehiscence with doi:10.1503/cmaj.180799/-/DC1). These findings were compatible erosion of bony and membranous sections (arrows) in a 54-year-old man with with cholesteatoma with lateral semicircular canal dehiscence. cholesteatoma. Note: *the malleus handle; +second genu of the facial nerve; During surgery, we noted that the osseous and membranous por- dotted lines define the tympanic segment of the facial nerve. -

(12) United States Patent (10) Patent No.: US 9,156,822 B2 Jin Et Al
US009 156822B2 (12) United States Patent (10) Patent No.: US 9,156,822 B2 Jin et al. (45) Date of Patent: Oct. 13, 2015 (54) FUNCTIONALLY SELECTIVE LIGANDS OF (2013.01); A61K 45/00 (2013.01); C07D DOPAMINED, RECEPTORS 215/20 (2013.01); C07D 217/24 (2013.01); (75) Inventors: Jian Jin, Chapel Hill, NC (US); Bryan C07D 231/56 (2013.01); C07D 235/26 Roth. Durham, NC (US); Stephen Frye, (2013.01); C07D 24I/04 (2013.01); C07D Chapel Hill, NC (US) 277/62 (2013.01); C07D 295/088 (2013.01); (73) Assignee: The University of North Carolina at of 6. 4. ES E. Chapel Hill, Chapel Hill, NC (US) ( .01); ( .01): s s 413/12 (2013.01); C07D 413/14 (2013.01); (*) Notice: Subject to any disclaimer, the term of this C07D 417/12 (2013.01); C07D 471/04 patent is extended or adjusted under 35 (2013.01) U.S.C. 154(b) by 150 days. (58) Field of Classification Search (21) Appl. No.: 13/807,347 CPC. A61 K31/551; C07D 401/02: C07D401/14: C07D 417/14: CO7D 471/04 (86). PCT No.: PCT/US2O11?042734 USPC ........................................... 514/218; 540/575 S371 (c)(1), See application file for complete search history. (2), (4) Date: Feb. 15, 2013 (87) PCT Pub. No.: WO2012/003418 (56) References Cited PCT Pub. Date: Jan. 5, 2012 U.S. PATENT DOCUMENTS (65) Prior Publication Data 5,006,528 A 4, 1991 Oshiro et al. 6,352,981 B1* 3/2002 Treiber et al. ................. 514, 183 US 2013/0137679 A1 May 30, 2013 7,160,888 B2 1/2007 Johnson et al. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX