Quantification of Background Cell-Free DNA to Further Refine Transplant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natera Announces Publication of Analytical Validation Study Demonstrating Superior Precision of Its Kidney Transplant Rejection Assay
Natera Announces Publication of Analytical Validation Study Demonstrating Superior Precision of Its Kidney Transplant Rejection Assay February 22, 2019 Core Technology Delivers Superior Analytical Performance, Underpins Outstanding Clinical Performance SAN CARLOS, Calif., Feb. 22, 2019 /PRNewswire/ -- Natera, Inc. (NASDAQ: NTRA), a leader in cell-free DNA, today announced analytical validation study results to be published online in the journal Transplantation demonstrating the superior performance of its donor-derived cell-free DNA (dd-cfDNA) test for detecting active rejection in kidney transplant recipients.1 The study showed superior assay precision with coefficient of variation up to five times better than previously published studies.1,2 Natera's analytical validation was based on the analysis of 1,064 replicate samples from both related and non-related donor-recipient pairs. Conducted according to rigorous guidelines from the Clinical & Laboratory Standards Institute, the validation study measured key properties of the assay, including lower limit of detection, linearity, and precision. The assay's precision was particularly strong, showing a coefficient of variation up to five times better than that of a competitive dd-cfDNA assay (1.85% vs. 9.2% within run; 1.99% vs. 4.5% across runs) in repeatability and reproducibility studies.1,2 Previously published analytical studies using other dd-cfDNA assays did not include related donor-recipient cases (such as parents or siblings), which is notable given the technical challenge of differentiating DNA patterns from close relatives. It has been estimated that 52 percent of live kidney donations originate from biologically related donors.3 Natera has leveraged its deep experience using single-nucleotide polymorphism (SNP)-based methods to analyze fetal DNA in maternal blood to achieve high accuracy in these cases. -

Now Quantify the Risk E a Cfdna Background Check Everytime
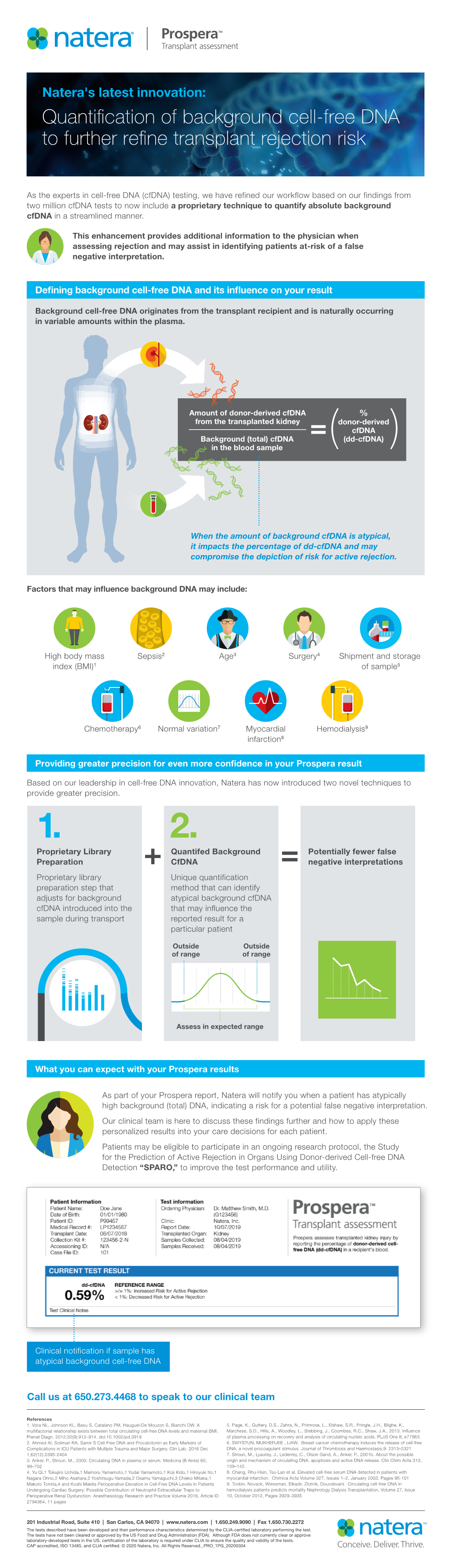
TM Prospera Now quantify the risk e A cfDNA background check everytime May 2020 How quantifying background cell-free DNA (cfDNA) is raising the bar for precision in rejection assessment Introduction Non-invasive monitoring using cell-free DNA (cfDNA) technology is an established method for distinguishing between patient DNA and non-patient DNA that is also in the bloodstream, such as cfDNA from a fetus (prenatal), tumor (oncology), or donor (transplantation).1–16 Donor-derived cfDNA (dd-cfDNA) is a proven biomarker in kidney and heart transplantation for identifying active rejection.1–6,13–16 Existing commercial assays report dd-cfDNA results as a percentage of total cfDNA. However, results reported in this manner may not provide a full picture of rejection risk due to background cfDNA levels that can be affected by many factors. Atypically high levels of recipient cfDNA may lead to a decreased dd-cfDNA proportion, and a potential false negative interpretation; less frequently, atypically low cfDNA levels can lead to false positive results. Natera’s ProsperaTM transplant assessment test is powered by highly optimized, proprietary cfDNA technology. It has now been enhanced with an exclusive technique, making Prospera the first test of its kind to quantify absolute background cfDNA. This enhancement benefits physicians by identifying patients with atypical background cfDNA levels – thereby flagging them as at-risk for false-negative reporting and potentially missed rejections. Prospera: Now quantify the risk – A cfDNA background check everytime May 2020 1 More? Less? Why the amount of background Amount of donor-derived cfDNA % cfDNA matters from the transplanted kidney donor-derived cfDNA Background (total) cfDNA = (dd-cfDNA) Background cfDNA originates ( ) in the blood sample from the transplant recipient and is naturally occurring in variable amounts within the plasma. -

Prospera™ Precision— from the Experts of Cell-Free DNA Testing
Prospera™ precision— from the experts of cell-free DNA testing New! From Natera Covered by Medicare Introducing Prospera Prospera is powered by highly optimized, proprietary cell-free DNA (cfDNA) technology. As part of your tool kit, Prospera assesses all types of kidney transplant rejection2 with great precision.1,3 Covered by Medicare for all kidney transplant recipients Simpler and less invasive than biopsy More sensitive and specific than current assessment tools across all types of rejection2,4,5 Up to 5x less variability than first-generation donor-derived cell-free DNA technology1,3 The need for something better. Experts in cell-free DNA. For transplant clinicians treating, monitoring and managing patients across the entire transplant continuum— Optimized for transplantation. To improve the management of organ transplantation, cancer, and reproductive health, Natera is the goal is earlier, accurate detection of rejection that harnessing the power of cfDNA from a single blood sample and a methodology that uses single-nucleotide enables treatment to promote longer graft life. polymorphisms (SNPs) for non-invasive testing. Current tools to monitor rejection are limited as they risk detecting rejection late or not at all, Optimized Organ Transplantation platform which often leads to chronic active antibody-mediated rejection (ABMR) and eventual graft Highest performing dd-cfDNA test failure. The lack of an effective tool to identify clinical and subclinical antibody-mediated and T cell-mediated rejection (TCMR) presents a critical challenge to the clinician. Applied Oncology experience First custom-built circulating-tumor DNA test Built Women’s Health foundation Pioneered SNP-based technology to a broad prenatal-testing product suite TRANSPLANT PATIENT CARE TOOLKIT An established methodology using a validated biomarker Cell-free DNA (cfDNA) technology is an established methodology in prenatal, oncology and transplantation applications.3,5,7-12 Further, cfDNA is a proven biomarker in kidney and heart transplantation 1-5,7,13-17 for identifying active rejection. -

June 4–9, 2021
THE SCIENCE OF TOMORROW STARTS TODAY ATC2021 VirtualCONNECT atcmeeting.org JUNE 4–9, 2021 Registration Brochure & Scientific Program DISCOUNTED REGISTRATION DEADLINE MAY 5, 2021 #ATC2021VirtualConnect ATC2021 VirtualCONNECT All-New Enhanced Experience! We are excited to announce ATC 2021 Virtual Live Connect, an all-new, completely enhanced virtual Broadcast Dates: meeting experience. Gain immediate access to innovators in the field and have your voice heard June 4 – 9, 2021 through various types of interaction Real-Time Interactivity Over 200 Education Credit The 2021 program will provide ample and Contact Hours opportunities for real-time interactivity through: ATC provides CME, ANCC, ACPE, and ABTC credits/contact hours. Yearlong access allows • Live Video Discussions you to take advantage of the over 200 • Invigorating Q&A Discussions Post- credits/contact hours available. This is the Presentation most credits/contact hours ATC has ever been • Live Presentations by Abstract Presenters able to provide! • Engaging, Unconventional Networking Breaks Continue to check the ATC website for final • Live Symposia Presentations credit/contact hour details, www.atcmeeting.org. Mobile Responsive Access In-Depth Symposia Included You’ll be able to access Virtual Connect on-the-go, in Virtual Connect earn your education credit or contact hours, hear Included with ATC 2021 Virtual Connect are the latest innovations, and build your professional 9 In-Depth Symposia. These symposia will be network – all from the comfort and safety of your live broadcasted on Friday, June 4, 2021, and home or office. then available in the OnDemand format until June 3, 2022. Yearlong Access to OnDemand Content Time Zone Schedule of By registering to attend ATC 2021 Virtual Connect, Program – Eastern Time Zone you also will gain yearlong access to all Live The program schedule is built in Eastern Time Broadcast sessions available in an OnDemand Zone. -

Natera Presents New Data at the 2020 SITC Annual Meeting Highlighting Applications of Personalized Ctdna Testing in Immunotherapy Treatment Monitoring
Natera Presents New Data at the 2020 SITC Annual Meeting Highlighting Applications of Personalized ctDNA Testing in Immunotherapy Treatment Monitoring November 10, 2020 Clinical case studies demonstrate the performance of the Signatera™ test in cancer patients receiving immunotherapy SAN CARLOS, Calif., Nov. 10, 2020 /PRNewswire/ -- Natera, Inc. (NASDAQ: NTRA), a pioneer and global leader in cell-free DNA testing, today announced it will present new immunotherapy data on its personalized and tumor-informed molecular residual disease (MRD) assay, Signatera, at the Society for Immunotherapy of Cancer (SITC) 35th Anniversary Annual Meeting, taking place November 9-14, 2020. Natera will present four posters demonstrating the ability of Signatera to monitor response to immunotherapy in colorectal and breast cancer. "Tumor-informed circulating tumor DNA tests have broad clinical utility across cancer types," said Georges Azzi, MD, oncologist at Holy Cross Health in Ft. Lauderdale, Florida and co-author of one of the posters presented. "I am excited to partner with Natera to further explore early indications of treatment response, or lack thereof, using this novel technology." "We have consistently demonstrated the clinical utility of MRD testing with Signatera to help patients detect recurrence earlier, and we are now seeing evidence of Signatera's ability to effectively monitor immunotherapy treatment," said Alexey Aleshin, MD, Natera's Senior Medical Director of Oncology. "The studies we are presenting at SITC showcase Signatera's broadened -

Personalized Circulating Tumor DNA Analysis As a Predictive Biomarker in Solid Tumor Patients Treated with Pembrolizumab Reprinted from Nature Cancer Scott V
The international journal of science nature cancer Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab Reprinted from Nature Cancer Scott V. Bratman 1,2,3,9, S. Y. Cindy Yang1,3,9, Marco A. J. Iafolla3,4, Zhihui Liu3,5, pp. 1–9, August 2020 Aaron R. Hansen3,4, Philippe L. Bedard 3,4, Stephanie Lheureux3,4, Anna Spreafico3,4, Albiruni Abdul Razak3,4, Svetlana Shchegrova6, Maggie Louie6, Paul Billings6, © 2020 Springer Nature Limited. All rights Bernhard Zimmermann 6, Himanshu Sethi6, Alexey Aleshin6, Dax Torti7, Kayla Marsh7, reserved. Jenna Eagles7, Iulia Cirlan 3, Youstina Hanna3, Derek L. Clouthier3, Scott C. Lien3,8, Pamela S. Ohashi3,8, Wei Xu3,5, Lillian L. Siu 3,4 and Trevor J. Pugh 1,3,7 1 Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada. 2Department of Radiation Oncology, University of Toronto, Toronto, Ontario, Canada. 3Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada. 4Division of Medical Oncology, Department of Medicine, University of Toronto, Toronto, Ontario, Canada. 5Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada. 6Natera, Inc., San Carlos, CA, USA. 7Ontario Institute of Cancer Research, Toronto, Ontario, Canada. 8Department of Immunology, University of Toronto, Toronto, Ontario, Canada. 9These authors contributed equally: Scott V. Bratman, S. Y. Cindy Yang. e-mail: [email protected]; [email protected] Nature | www.nature.com/natcancer ARTICLES https://doi.org/10.1038/s43018-020-0096-5 Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab Scott V. -

Natera Reports Third Quarter 2020 Financial Results
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event reported): November 5, 2020 Natera, Inc. (Exact name of registrant as specified in its charter) Delaware 001-37478 01-0894487 (State or other jurisdiction (Commission (IRS Employer of incorporation) File Number) Identification No.) 201 Industrial Road, Suite 410 San Carlos, California 94070 (Address of principal executive offices, including zip code) (650) 249-9090 (Registrant’s telephone number, including area code) N/A (Former name or former address, if changed since last report) Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2): ☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) ☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) ☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) ☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Securities registered pursuant to Section 12(b) of the Act: Trading Name of each exchange Title of each class Symbol(s) on which registered Common Stock, par value $0.0001 per share NTRA Nasdaq Stock Market LLC (Nasdaq Global Select Market) Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). -
Case 1:20-Cv-00692-ADA Document 1 Filed 06/01/20 Page 1 of 41
Case 1:20-cv-00692-ADA Document 1 Filed 06/01/20 Page 1 of 41 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF TEXAS WACO DIVISION Ravgen, Inc., Plaintiff, Civil Action No. 6:20-cv-451 v. JURY TRIAL DEMANDED Natera, Inc. and NSTX, Inc., Defendants. COMPLAINT FOR PATENT INFRINGEMENT Plaintiff Ravgen, Inc. (“Ravgen”), for its Complaint against Defendants Natera, Inc. and NSTX, Inc. (collectively “Defendants”), hereby alleges as follows: NATURE OF THE ACTION 1. This is a civil action for infringement of United States Patent Nos. 7,727,720 (the “’720 Patent”) and 7,332,277 (the “’277 Patent”) (collectively the “Patents-in-Suit”), arising under the Patent Laws of the United States, 35 U.S.C. §§ 271, et seq. THE PARTIES 2. Plaintiff Ravgen is a Delaware corporation with its principal place of business at 9241 Rumsey Rd., Columbia, MD 21045. Ravgen is a pioneering diagnostics company that focuses on non-invasive prenatal testing. Ravgen has spent millions of dollars researching and developing novel methods for the detection of cell-free DNA to replace conventional, invasive procedures. Ravgen’s innovative cell-free DNA technology has various applications, including non-invasive prenatal and other genetic testing. Those efforts have resulted in the issuance of several patents, including the Patents-in-Suit. Case 1:20-cv-00692-ADA Document 1 Filed 06/01/20 Page 2 of 41 3. Defendant Natera, Inc. is a Delaware corporation with its principal place of business at 201 Industrial Road, San Carlos, California 94070. (Ex. 9 at 1 (Texas Secretary of State report for Natera, Inc.).) Natera, Inc. -

Cell-Free Fetal DNA Testing (For Tennessee Only)
UnitedHealthcare® Community Plan Medical Policy Cell-Free Fetal DNA Testing (for Tennessee Only) Policy Number: CS085TN.O Effective Date: May 1, 2021 Instructions for Use Table of Contents Page Related Community Plan Policy Application.......................................................................................... 1 • Chromosome Microarray Testing (Non-Oncology Coverage Rationale ........................................................................... 1 Conditions) (for Tennessee Only) Definitions ........................................................................................... 2 Applicable Codes .............................................................................. 3 Commercial Policy Description of Services ..................................................................... 8 • Cell-Free Fetal DNA Testing Clinical Evidence ............................................................................... 9 U.S. Food and Drug Administration ..............................................31 Centers for Medicare and Medicaid Services .............................31 References .......................................................................................31 Policy History/Revision Information..............................................37 Instructions for Use .........................................................................37 Application This Medical Policy applies to Medicaid only plans in the state of Tennessee. Coverage Rationale DNA-based noninvasive prenatal tests of fetal aneuploidy are -

2018 Natera Annual Report
2018 ANNUAL REPORT UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2018 OR TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number: 001-37478 NATERA, INC. (Exact Name of Registrant as Specified in Its Charter) Delaware 01-0894487 (State or Other Jurisdiction of Incorporation or Organization) (I.R.S. Employer Identification No.) 201 Industrial Road, Suite 410 San Carlos, CA 94070 (Address of Principal Executive Offices) (Zip Code) (650) 249-9090 (Registrant’s Telephone Number, Including Area Code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered The NASDAQ Stock Market LLC Common Stock, par value $0.0001 per share (NASDAQ Global Select Market) Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Act. Yes No Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

International Society for Prenatal Diagnosis Recommends Cfdna Screening in Twin Pregnancies
International Society for Prenatal Diagnosis Recommends cfDNA Screening in Twin Pregnancies October 30, 2020 Recommends incorporating zygosity, a capability unique to the Panorama® test, into clinical practice SAN CARLOS, Calif., Oct. 30, 2020 /PRNewswire/ -- Natera, Inc. (NASDAQ: NTRA), a pioneer and global leader in cell-free DNA (cfDNA) testing, applauds the recent International Society for Prenatal Diagnosis (ISPD) position statement recommending the use of cfDNA screening in twin pregnancies. The position statement can be found here. The ISPD position statement endorses cfDNA screening for autosomal trisomies in twin pregnancies and recommends that laboratories incorporate zygosity into the interpretation of non-invasive prenatal testing (NIPT) results. The statement also highlights the ability of SNP-based NIPT to determine zygosity and individual fetal fractions in dizygotic pregnancies.1 "ISPD has now echoed the ACOG-SMFM position recognizing the clinical value of NIPT for twin pregnancies, citing high sensitivity and positive predictive values," said Herman Hedriana, MD, MFM, Professor of Clinical Obstetrics and Gynecology and Director, Maternal-Fetal Medicine at UC Davis Health. "Both sets of recommendations highlight the utility of zygosity, an attribute unique to Natera's Panorama test, which can inform triaging of high-risk twin pregnancies to specialists." "Given the Panorama test's unique ability to determine zygosity and individual fetal fraction in dizygotic pregnancies, these guidelines provide a good opportunity for us to expand our market leadership in NIPT," said Ramesh Hariharan, General Manager for Natera's Women's Health business." About Panorama® Panorama reveals a baby's risk for severe genetic disorders as early as nine weeks into pregnancy. -

Innovations in Transplant Oncology
Innovations in transplant oncology Explore new technologies to transform the management of cancer patients in organ transplantation Join us in understanding how SignateraTM and ProsperaTM can improve decision-making at the complex intersection of organ transplantation and oncology. Natera’s innovations in transplant oncology Advances in Cell-Free DNA Testing Transform the management of cancer patients in organ transplantation Pre-Transplant Evaluation Post-Transplant Monitoring • A signicant number of patients • Transplant recipients also require evaluated for transplantation have immunosuppressive medications a history of cancer. to avoid rejection, which can • Patients with end-stage renal disease increase the risk of new or have a 20% higher rate of colorectal recurring cancers. cancer than the general population.1 • A history of cancer makes it difcult for patients to receive a transplant due to the uncertain risk of recurrence. Rejection vs Cancer and Infection SignateraTM ProsperaTM SignateraTM Cancer Recurrence Rejection Monitoring Cancer Recurrence Monitoring Monitoring A Balance of Immunosuppression CONCERT STUDY PARC STUDY SIGNAL STUDY Colorectal Neoplasm in Candidates Enlisting Prospera in Renal Allograft Signatera in Liver Cancer for Renal Transplantation Recipients with Cancer An observational study of A study to accelerate clearance for renal transplantation A study to understand how Signatera in patients with liver in molecular residual disease (MRD)-negative patients malignancies and cancer therapy cancer to determine molecular with a history of colorectal cancer affect background cell-free DNA residual disease (MRD) rates levels in renal allograft recipients. before and after liver transplant. The goal of Natera’s Innovation in Transplant Oncology initiative is to understand how Signatera and Prospera can be used to improve decision-making at the complex intersection of organ transplantation and oncology, and to respond to the unmet needs within these communities.