Optimizing Plant Density and Nitrogen Application to Manipulate Tiller Growth and Increase Grain Yield and Nitrogen-Use Efficiency in Winter Wheat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MEASURING & MONITORING Plant Populations
MEASURING & MONITORING Plant Populations COVER PHOTOS The cover landscape photo and the picture of the two people sampling were taken by Daniel Salzer. Both photos were taken at The Nature Conservancy's Katharine Ordway Sycan Marsh Preserve. The individuals shown sampling in the small photo are Rob Lindsay and Linda Poole Rexroat, both are TNC employees. The inset flower photo was taken by Linda M. Hardie, and shows grass-widows (Sisyrinchium douglasii), at the Nature Conservany’s Tom McCall Preserve at Rowena Crest. Copies available from: Bureau of Land Management National Business Center BC-650B P.O. Box 25047 Denver, Colorado 80225-0047 MEASURING & MONITORING Plant Populations AUTHORS: Caryl L. Elzinga Ph.D. Alderspring Ecological Consulting P.O. Box 64 Tendoy, ID 83468 Daniel W. Salzer Coordinator of Research and Monitoring The Nature Conservancy of Oregon 821 S.E. 14th Avenue Portland, OR 97214 John W. Willoughby State Botanist Bureau of Land Management California State Office 2135 Butano Drive Sacramento, CA 95825 This technical reference represents a team effort by the three authors. The order of authors is alphabetical and does not represent the level of contribution. Though this document was produced through an interagency effort, the following BLM numbers have been assigned for tracking and administrative purposes: BLM Technical Reference 1730-1 BLM/RS/ST-98/005+1730 MEASURING AND MONITORING PLANT POPULATIONS ACKNOWLEDGEMENTS The production of this document would not have been possible without the help of many individuals. Phil Dittberner of the Bureau of Land Management's National Applied Resource Sciences Center (NARSC) coordinated the effort for BLM. -

Effects of Plant Density and Sowing Date on the Growth, Flowering and Quantity of Essential Oil of Calendula Officinalis L
Journal of Medicinal Plants Research Vol. 5(20), pp. 5110-5115, 30 September, 2011 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2011 Academic Journals Full Length Research Paper Effects of plant density and sowing date on the growth, flowering and quantity of essential oil of Calendula officinalis L. Ahmad Reza Berimavandi 1*, Davood Hashemabadi 2, Mohammad Vali Facouri Ghaziani 2 and Behzad Kaviani 2 1Department of Agronomy, Rasht Branch, Islamic Azad University, Rasht, Iran. 2Department of Horticultural Science, Rasht Branch, Islamic Azad University, Rasht, Iran. Accepted 6 July, 2011 Pot marigold ( Calendula officinalis L.) is a medicinal and ornamental plant. In an experiment which was designed in factorial on the basis of completely randomized block design with 3 replicates in 36 plots. The effects of plant density (20, 40, 60 and 80 plants/m 2- factor A) and sowing date (April 19 and 29, and May 10, 2010- factor B) were evaluated on the growth, flowering and essential oil of C. officinalis L. Studies were carried out on experimental field in Islamic Azad University, Rasht, Iran. Investigated characteristics were: plant dry weight, flower number/plant, branch number/plant, flower dry weight, flower yield/area unit, and quantity of essential oil. Maximum of plant dry weight (35.67 g), flower number/plant (25.88), branch number/plant (9.44), flower dry weight (3.72 g), and amount of essential oil/100 g dried flower (0.16 ml) were obtained at the 20 plants/m 2, while maximum of flower dry weight (132.07 g), and amount of essential oil (0.19 ml), both per area unit were obtained at the 60 plants/m2. -

The Impact of Plant Density and Spatial Arrangement on Light Interception on Cotton Crop and Seed Cotton Yield: an Overview
CHAPEPA et al. Journal of Cotton Research (2020) 3:18 Journal of Cotton Research https://doi.org/10.1186/s42397-020-00059-z REVIEW Open Access The impact of plant density and spatial arrangement on light interception on cotton crop and seed cotton yield: an overview CHAPEPA Blessing1*, MUDADA Nhamo2 and MAPURANGA Rangarirai3 Abstract Light attenuation within a row of crops such as cotton is influenced by canopy architecture, which is defined by size, shape and orientation of shoot components. Level of light interception causes an array of morpho-anatomical, physiological and biochemical changes. Physiological determinants of growth include light interception, light use efficiency, dry matter accumulation, duration of growth and dry matter partitioning. Maximum light utilization in cotton production can be attained by adopting cultural practices that yields optimum plant populations as they affect canopy arrangement by modifying the plant canopy components. This paper highlights the extent to which spatial arrangement and density affect light interception in cotton crops. The cotton crop branches tend to grow into the inter-row space to avoid shade. The modification of canopy components suggests a shade avoidance and competition for light. Maximum leaf area index is obtained especially at flowering stage with higher populations which depicts better yields in cotton production. Keywords: Light interception, Plant populations, Spatial arrangement, Canopy architecture Introduction Bai et al. (2016), the extent and pattern of attenuation Plants contain the green pigment chlorophyll and they are determined by the architecture of the canopy and require light energy from the sun to photosynthesize cotton with columnar-type canopies exhibits an open and to grow. -

Seed Dispersal in Corn Crops Under Mediterranean Conditions Judit Barroso, Dionisio Andu´Jar, Carolina San Martı´N, Ce´Sar Ferna´Ndez-Quintanilla, and Jose´ Dorado*
Weed Science 2012 60:34–41 Johnsongrass (Sorghum halepense) Seed Dispersal in Corn Crops under Mediterranean Conditions Judit Barroso, Dionisio Andu´jar, Carolina San Martı´n, Ce´sar Ferna´ndez-Quintanilla, and Jose´ Dorado* Natural dissemination of johnsongrass seeds as well as the effect of combine harvesting on this process were studied in corn fields. The estimation of natural dispersal was carried out by two different methods, collecting seeds throughout the season using seed traps and sampling soil–surface seed abundance before harvest using a vacuum device. Both methods showed the same dispersal pattern. A minimum of 84.6% was dispersed in the first 2 m from the focus and a maximum of 1.6% was dispersed beyond the first 5 m. An average of 76.3% of these dispersed seeds were lost or buried after shedding but before harvest. Seed dispersal by the combine harvester was estimated from the difference between soil–surface seed abundance in the same sites pre and postharvest. Although the quantity of seeds dispersed by the combine was similar to those dispersed by natural factors, dispersal distances were significantly higher. Around 90% of the dispersed seeds were found in the first 5 m forward and backward of the combine direction from the infestation source, and 1.6% of the seeds were found beyond 22 m forward and 10 m backward of the combine direction from the infestation source. A large proportion of the seeds dispersed were dormant or not viable. It is concluded that the major role of sexual reproduction in johnsongrass population dynamics may be to spread the risks, promoting dispersal in time and space. -
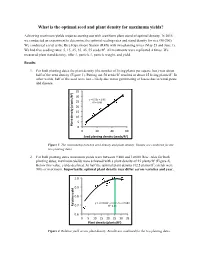
What Is the Optimal Seed and Plant Density for Maximum Yields?
What is the optimal seed and plant density for maximum yields? Achieving maximum yields requires starting out with a uniform plant stand of optimal density. In 2015 we conducted an experiment to determine the optimal seeding rates and stand density for rice (M-206). We conducted a trial at the Rice Experiment Station (RES) with two planting times (May 25 and June 1). We had five seeding rates: 5, 15, 25, 35, 45, 55 seeds/ft2. All treatments were replicated 4 times. We measured plant stand density, tiller #, panicle #, panicle weight, and yield. Results 1. For both planting dates the plant density (the number of living plants per square foot) was about half of the seed density (Figure 1). Putting out 50 seeds/ft2 resulted in about 25 living plants/ft2. In other words, half of the seed were lost – likely due to not germinating or losses due to wind, pests and disease. 35 ) 2 30 y = 0.53x + 1.96 25 R² = 0.98 20 15 10 5 Plant density (plants// 0 0 20 40 60 2 Seed planng density (seeds/ ) Figure 1. The relationship between seed density and plant density. Results are combined for the two planting dates. 2. For both planting dates maximum yields were between 9,800 and 10,000 lb/ac. Also for both planting dates, maximum yields were achieved with a plant density of 25 plants/ft2 (Figure 2). Below this value, yields declined. At half the optimal plant density (12.5 plants/ft2) yields were 90% of maximum. Importantly, optimal plant density may differ across varieties and year. -

The Effect of Plant Population Densities on Growth, Yield and Yield Components of Two Spring Rapeseed Cultivars
The effect of plant population densities on growth, yield and yield components of two spring rapeseed cultivars H. Ozer Faculty of Agriculture, Ataturk University, Erzurum, Turkey ABSTRACT Rapeseed (Brassica napus L.) is an important alternate oilseed crop in eastern Anatolia, Turkey. No information on plant density for rapeseed is available in this region. Therefore a study was initiated to investigate the effects of spacings between rows and spacings within rows on the yield and agronomic characteristics of two genotypes of spring rapeseed (Tower and Lirawell) in Erzurum, eastern Anatolia, during 1994 and 1995. The effects of spacings between or within rows on the yield and yield components of Tower and Lirawell, two cultivars of Brassica napus L., were studied for 2 years in Erzurum, Turkey. Rows were spaced at 15, 30 and 45 cm. Spacings within rows were 5, 10 and 15 cm. The results of this study suggested that seed yield was significantly affected by spacings between rows but not by spacings within rows, and that rape yields were higher at the narrow (15 cm) row spacing compared to the middle (30 cm) and wider (45 cm) spacings. Keywords: rapeseed; Brassica napus L.; plant density; row spacing; seed yield In general, Turkey has a suitable climate for many oil- The objective of this study was to evaluate the effects seeds. This is not completely true of eastern Anatolia of different spacings between or within rows on the ag- with low temperatures and short growing season, where ronomic characteristics of two genotypes of Brassica the oilseed crops usually perform poorly except sunflow- napus L., cv. -

„Effects of Plant Density, Interlighting, Light Intensity and Light Quality on Growth, Yield and Quality of Greenhouse Sweet Pepper“
Rit LbhÍ nr. 30 „Effects of plant density, interlighting, light intensity and light quality on growth, yield and quality of greenhouse sweet pepper“ FINAL REPORT Christina Stadler 2010 Rit LbhÍ nr. 30 ISBN 9789979881052 „Effects of plant density, interlighting, light intensity and light quality on growth, yield and quality of greenhouse sweet pepper“ FINAL REPORT Christina Stadler Landbúnaðarháskóli Íslands Nóvember 2010 Final report of the research project „Effects of plant density, interlighting, light intensity and light quality on growth, yield and quality of greenhouse sweet pepper“ Duration: 15/07/2008 – 15/10/2009 Project leader: Landbúnaðarháskóla Íslands Reykjum Dr. Christina Stadler 810 Hveragerði Email: [email protected] Tel.: 433 5312 (Reykir), 433 5249 (Keldnaholt) Mobile: 843 5312 Collaborators: Magnús Ágústsson, Bændasamtökum Íslands Hjalti Lúðvíksson, Frjó Quatro ehf. Knútur Ármann, Friðheimum Sveinn Sæland, Espiflöt Dr. Mona-Anitta Riihimäki, Martens Trädgårdsstiftelse, Finnland Project sponsor: Samband Garðyrkjubænda Bændahöllinni við Hagatorg 107 Reykjavík Framleiðnisjóður landbúnaðarins Hvanneyrargötu 3 Hvanneyri 311 Borgarnes Table of contents List of figures III List of tables V Abbreviations VI 1 SUMMARY 1 2 INTRODUCTION 3 3 MATERIALS AND METHODS 6 3.1 Greenhouse experiment 6 3.2 Lighting regimes 8 3.3 Measurements, sampling and analyses 9 3.4 Statistical analyses 10 4 RESULTS 11 4.1 Environmental conditions for growing 11 4.1.1 Solar irradiation 11 4.1.2 Illuminance 11 4.1.3 Air temperature 13 4.1.4 Soil temperature -

How Does Sowing Rate Affect Plant and Stem Density, Forage Yield, and Nutritive Value in Glyphosate-Tolerant Alfalfa?
agronomy Article How Does Sowing Rate Affect Plant and Stem Density, Forage Yield, and Nutritive Value in Glyphosate-Tolerant Alfalfa? Marisol T. Berti * and Dulan Samarappuli Department of Plant Sciences, North Dakota State University, Fargo, ND 58105, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-701-231-6110 Received: 31 July 2018; Accepted: 28 August 2018; Published: 30 August 2018 Abstract: The recommended sowing rate of alfalfa (Medicago sativa L.) is about 10 kg pure live seed ha−1, but it is debated if increasing the sowing rate enhances forage yield and quality in the sowing year. This study was conducted to: (i) determine the optimal sowing rate to maximize forage yield; and (ii) determine the relationship between plant and stem density with forage yield and nutritive value. Experiments were conducted at three sites in North Dakota between 2013 and 2016. Six sowing rates [1, 5, 10, 15, 20, and 25 kg ha−1 pure live seed (PLS)] were evaluated. Results indicated that total forage yield in the sowing year was lower only with the lowest sowing rate. Maximum total forage yield in the sowing, first, second, and third production years was obtained with 73, 52, 37, and 36 plants m−2 and 575, 495, 435, and 427 stems m−2, respectively. In the sowing and first production year, both plant and stem density predicted forage yield similarly. In older stands, stem density predicted forage yield slightly better. Forage nutritive value was similar among sowing rates indicating an increase in sowing rate does not enhance forage nutritive value. -

Diversity, Density, and Development of Early Vegetation in a Small Clear-Cut Environment
United States Department Diversity, Density, and Development of of Agriculture Forest Service Early Vegetation in a Small Clear-cut Pacific Southwest Environment Research Station http://www.psw.fs.fed.us/ Research Paper PSW-RP-239 Philip M. McDonald Publisher: Pacific Southwest Research Station Albany, California Forest Service Mailing address: U.S. Department of Agriculture PO Box 245, Berkeley CA 94701-0245 Abstract 510 559-6300 McDonald, Philip M. 1999. Diversity, density, and development of early vegetation in a small clear-cut environment. Res. Paper PSW-RP-239. Albany, CA: Pacific Southwest Research Station, Forest Service, http://www.psw.fs.fed.us U.S. Department of Agriculture; 22 p. September 1999 On a high quality site in northern California, frequency, density, foliar cover, and height were measured on every plant species present in an 8-acre clear-cut opening each year from 1976 through 1980. Plant species numbered 71, although no more than 62 were present during a given year. Categories of vegetation with the most plants per acre initially were shrubs, annuals and biennials, and ferns; at the end of the study the categories were annuals and biennials, graminoids, and shrubs. At the beginning of the study, bracken fern provided the highest amount of foliar cover, whereas 5 years later shrubs and annuals and biennials provided the most cover. Hardwoods and graminoids were the tallest categories of vegetation. At the study’s end density was 112,408 plants per acre, foliar cover was 17,433 ft2 per acre and the tallest plants were 3.5 to 4.9 feet tall. -

MF3389 Plant Density and Yield Environment Interaction
Corn Fact Sheet Series Plant Density and Yield Environment Interaction Plant density is a main corn yield factor (along with determined until after pollination and early grain fill ear number per unit area, grain number per ear, and due to relative success of fertilization and degree of early grain weight) that affects final yield. Final plant density abortion of ovules. is the result of selecting an optimal seeding rate, which Optimal seeding rates may need to be adjusted for is a main crop management component influencing the irrigated corn if fertilizer or irrigation rates are sharply economic return for corn. Compared to other crops, increased or decreased. For example, research at the such as sorghum, wheat, and soybeans, seeding rate Irrigation Experiment Field near Scandia showed that if and final plant density at harvest largely influences fertilizer rates are increased, seeding rates also have to be attainable corn yield. Unlike other cereals, corn does not increased to realize the maximum yield benefit. have the same ability to compensate when plants are New Review Analysis missing. Modern corn hybrids usually do not produce tillers and the plant commonly generates one ear per A database was constructed from 124,374 plant plant at current optimum plant densities. Commercially density observations conducted from 2000 through important corn hybrids are highly dependent on plant 2014 in 22 U.S. states and two Canadian provinces. density. These trials were conducted in a split-plot arrangement The optimal seeding rate depends on the genotype with two to three replicates at each location. Plant (G), environment (E), and management practices (M). -

Effects of Environment and Planting Density on Plant Stature, Flowering Time, and Ear Set in IBM Populations of Maize Sara Jane Helland Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2012 Effects of environment and planting density on plant stature, flowering time, and ear set in IBM populations of maize Sara Jane Helland Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Genetics Commons, and the Plant Sciences Commons Recommended Citation Helland, Sara Jane, "Effects of environment and planting density on plant stature, flowering time, and ear set in IBM populations of maize" (2012). Graduate Theses and Dissertations. 12340. https://lib.dr.iastate.edu/etd/12340 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Effects of environment and planting density on plant stature, flowering time, and ear set in IBM populations of maize by Sara Jane Helland A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Plant Breeding Program of Study Committee: Nick Lauter, Co-Major Professor Kendall Lamkey, Co-Major Professor Michael Muszynski Maria Salas-Fernandez Paul Scott Iowa State University Ames, Iowa 2012 ii TABLE OF CONTENTS ABSTRACT iii CHAPTER 1. GENERAL INTRODUCTION Introduction 1 Thesis Organization 3 Literature Review 4 Literature Cited 18 CHAPTER 2. GENETIC AND ENVIRONMENTAL CONTROL OF SHOOT ARCHITECTURE AND FLOWERING TIME TRAITS IN MAIZE IBMRILS GROWN IN ACROSS CORNBELT ENVIRONMENTS Abstract 30 Introduction 31 Materials and Methods 37 Results 44 Discussion 48 Acknowledgements 55 Literature Cited 55 CHAPTER 3. -

Planting Density Effects on Grow Rate, Biometric Parameters, And
agriculture Article Planting Density Effects on Grow Rate, Biometric Parameters, and Biomass Calorific Value of Selected Trees Cultivated as SRC Adam Kleofas Berbe´c* and Mariusz Matyka Department of Systems and Economics of Crop Production, Institute of Soil Science and Plant Cultivation-State Research Institute, Czartoryskich 8, 24-100 Puławy, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-81-4786-824 Received: 7 October 2020; Accepted: 25 November 2020; Published: 26 November 2020 Abstract: Agricultural land is mostly devoted to food production. Production of biomass is limited, as it competes for land with basic food production. To reduce land loss for growing food, biomass can be grown on marginal lands that are not usable for food production. The density of plantings have to be optimized to maximize yield potential. The presented study compares yield parameters end energy potential of six species of biomass plants (poplar, Siberian elm, black alder, white birch, boxelder maple, silver maple) cultivated in 18 planting densities from 3448 to 51,282 plants per hectare as short rotation coppice (SRC). Biomass yield parameters depended on both cultivated species and planting density. Green mass, dry mass, and shoot diameter was dropping with the increasing planting density for most tested species. Calculated yield of dry mass was dropping with increasing planting density for black alder, increasing for Siberian elm and boxelder maple. White birch and silver maple yields were optimal at moderate planting densities (25,000–30,000). White birch and boxelder maple had the highest average higher heating value (HHV). The optimal density of plantings should be chosen to best suit both the needs of cultivated species and to optimize the most important parameters of produced biomass.