Principles and Practice of PET/CT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Radiosynthesis and in Vivo Evaluation of a 18F-Labelled Styryl-Benzoxazole Derivative for Β-Amyloid Targeting
Author's Accepted Manuscript Radiosynthesis and in vivo Evaluation of a 18F- Labelled Styryl-Benzoxazole Derivative for β- Amyloid Targeting G.Ribeiro Morais, L. Gano, T. Kniess, R. Berg- mann, A. Abrunhosa, I. Santos, A. Paulo www.elsevier.com/locate/apradiso PII: S0969-8043(13)00292-3 DOI: http://dx.doi.org/10.1016/j.apradiso.2013.07.003 Reference: ARI6294 To appear in: Applied Radiation and Isotopes Received date: 9 January 2013 Revised date: 15 April 2013 Accepted date: 1 July 2013 Cite this article as: G.Ribeiro Morais, L. Gano, T. Kniess, R. Bergmann, A. Abrunhosa, I. Santos, A. Paulo, Radiosynthesis and in vivo Evaluation of a 18F- Labelled Styryl-Benzoxazole Derivative for β-Amyloid Targeting, Applied Radiation and Isotopes, http://dx.doi.org/10.1016/j.apradiso.2013.07.003 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Radiosynthesis and in vivo Evaluation of a 18F-Labelled Styryl-Benzoxazole Derivative for β-Amyloid Targeting G. Ribeiro Morais,1 L. Gano,1 T. Kniess,2 R. Bergmann,2 A. Abrunhosa,3 I. Santos,1 and A. Paulo1* 1Radiopharmaceutical Sciences Group, IST/ITN, Instituto Superior Técnico, Universidade Técnica de Lisboa, EN 10, 2686-953 Sacavem; 2Institute of Radiopharmacy, Helmholtz-Zentrum Dresden-Rossendorf e.V., POB 510119, D-01314 Dresden, Germany; 3Universidade Coimbra, ICNAS, Inst Nucl Sci Appl Saúde, P-3000 Coimbra, Portugal Corresponding e-mail: [email protected] Keywords: Alzheimer´s Disease/ β-Amyloid aggregation/ Molecular Imaging / Fluorine-18 Abstract The formation of β-amyloid deposits is considered a histopathological feature of Alzheimer´s disease (AD). -

Clinical Appropriateness Guidelines Positron Emission Testing, Other PET Applications, Including Oncologic Tumor Imaging Effective Date: April 21, 2014
Clinical Appropriateness Guidelines Positron Emission Testing, Other PET Applications, including Oncologic Tumor Imaging Effective Date: April 21, 2014 Proprietary and Confidential Date of Origin: 03/30/2005 Last reviewed: 11/14/2013 Last revised: 01/15/2013 Clinical Appropriateness Guidelines 8600 W Bryn Mawr Avenue South Tower - Suite 800 Chicago, IL 60631 P. 773.864.4600 Copyright © 2014. AIM Specialty Health. All Rights Reserved www.aimspecialtyhealth.com Table of Contents Administrative Guideline ..........................................................................................................3 Disclaimer ..............................................................................................................................................................3 Use of AIM’s Diagnostic Imaging Guidelines..........................................................................................................4 Multiple Simultaneous Imaging Requests ..............................................................................................................5 General Imaging Considerations ............................................................................................................................6 PET - Other PET Applications, Including Oncologic Tumor Imaging ......................................8 PET Bibliography ....................................................................................................................12 Table of Contents | Copyright © 2014. AIM Specialty Health. All Rights Reserved. -

Myocardial Perfusion Imaging in Coronary Artery Disease
Cor et Vasa Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/crvasa Přehledový článek | Review article Myocardial perfusion imaging in coronary artery disease Magdalena Kostkiewicza,b a Department of Cardiovascular Diseases, Jagiellonian University, Collegium Medicum, Hospital John Paul II, Krakow, Poland b Department of Nuclear Medicine, Hospital John Paul II, Jagiellonian University, Collegium Medicum, Krakow, Poland ARTICLE INFO SOUHRN Article history: Radionuklidové zobrazování perfuze myokardu (myocardial perfusion imaging, MPI) lze použít k prokázání Received: 22. 8. 2015 přítomnosti ischemické choroby srdeční (ICHS), stratifi kaci rizika i k vedení léčby pacientů s již potvrzeným Received in revised form: 26. 9. 2015 onemocněním. Uvedená metoda je schopna lokalizovat hemodynamicky významné stenózy koronárních Accepted: 28. 9. 2015 tepen i zhodnotit rozsah a závažnost jejich obstrukce podle přítomnosti a rozsahu defektů perfuze. Nor- Available online: 31. 10. 2015 mální výsledek MPI znamená nepřítomnost koronární obstrukce, a tedy i klinicky významného onemocnění. Předností vyšetření srdce metodou PET je oproti SPECT její vyšší prostorové a časové rozlišení i nižší radiační zátěž pacienta. Zdá se, že hybridní vyšetření srdce kombinací SPECT nebo PET s údaji z CT nabízí přesnější Klíčová slova: a spolehlivější diagnostické a prognostické informace o pacientech se středně vysokým rizikem rozvoje ICHS. Ischemická choroba srdeční V poslední době byl zaznamenán významný pokrok ve smyslu přesnější kvantifi kace průtoku krve myokar- PET dem a koronární průtokové rezervy. Několik studií rovněž prokázalo, že kombinace zobrazení apoptózy Radionuklidové zobrazování perfuze a tvorby matrixových metaloproteináz může být prospěšná při zobrazování nestabilních plátů a vyhledání myokardu skupin asymptomatických pacientů s vysokým rizikem, pro něž znamená vyšetření zobrazovací metodou SPECT největší přínos. -

LAS VEGAS PRODUCT CATALOG INGREDIENTS Full Page Ad for FINE PASTRY 11”X 8.5”
PRODUCT CATALOG LAS VEGAS chefswarehouse.com BAKING AND PASTRY FROZEN/RTB BREAD ...................12 BEVERAGES, GOAT CHEESE ............................21 CONDIMENTS BAKING JAM ..............................4 PIZZA SHELLS ...............................12 COFFEE AND TEA GOUDA.......................................21 AND JAMS TORTILLAS/WRAPS ......................12 HAVARTI.......................................22 BAKING MIXES ............................4 BAR MIXERS ................................17 CHUTNEY ....................................25 WRAPPERS ..................................12 JACK CHEESE .............................22 BAKING SUPPLIES .......................4 BITTERS .........................................17 GLAZES AND DEMI-GLAZES .......25 BROWNIES ..................................12 MASCARPONE ...........................22 COLORANTS ...............................4 CORDIAL ....................................17 KETCHUP .....................................25 CAKES ASSORTED ......................12 MISCELLANEOUS ........................22 CROISSANTS ...............................4 JUICE ...........................................17 MAYO ..........................................25 TARTS ...........................................13 MOUNTAIN STYLE ........................22 DÉCOR ........................................4 MISCELLANEOUS ........................17 MUSTARD ....................................25 COULIS ........................................13 MOZZARELLA ..............................22 EXTRACTS ....................................6 -

FIC-Prop-65-Notice-Reporter.Pdf
FIC Proposition 65 Food Notice Reporter (Current as of 9/25/2021) A B C D E F G H Date Attorney Alleged Notice General Manufacturer Product of Amended/ Additional Chemical(s) 60 day Notice Link was Case /Company Concern Withdrawn Notice Detected 1 Filed Number Sprouts VeggIe RotInI; Sprouts FruIt & GraIn https://oag.ca.gov/system/fIl Sprouts Farmers Cereal Bars; Sprouts 9/24/21 2021-02369 Lead es/prop65/notIces/2021- Market, Inc. SpInach FettucIne; 02369.pdf Sprouts StraIght Cut 2 Sweet Potato FrIes Sprouts Pasta & VeggIe https://oag.ca.gov/system/fIl Sprouts Farmers 9/24/21 2021-02370 Sauce; Sprouts VeggIe Lead es/prop65/notIces/2021- Market, Inc. 3 Power Bowl 02370.pdf Dawn Anderson, LLC; https://oag.ca.gov/system/fIl 9/24/21 2021-02371 Sprouts Farmers OhI Wholesome Bars Lead es/prop65/notIces/2021- 4 Market, Inc. 02371.pdf Brad's Raw ChIps, LLC; https://oag.ca.gov/system/fIl 9/24/21 2021-02372 Sprouts Farmers Brad's Raw ChIps Lead es/prop65/notIces/2021- 5 Market, Inc. 02372.pdf Plant Snacks, LLC; Plant Snacks Vegan https://oag.ca.gov/system/fIl 9/24/21 2021-02373 Sprouts Farmers Cheddar Cassava Root Lead es/prop65/notIces/2021- 6 Market, Inc. ChIps 02373.pdf Nature's Earthly https://oag.ca.gov/system/fIl ChoIce; Global JuIces Nature's Earthly ChoIce 9/24/21 2021-02374 Lead es/prop65/notIces/2021- and FruIts, LLC; Great Day Beet Powder 02374.pdf 7 Walmart, Inc. Freeland Foods, LLC; Go Raw OrganIc https://oag.ca.gov/system/fIl 9/24/21 2021-02375 Ralphs Grocery Sprouted Sea Salt Lead es/prop65/notIces/2021- 8 Company Sunflower Seeds 02375.pdf The CarrIngton Tea https://oag.ca.gov/system/fIl CarrIngton Farms Beet 9/24/21 2021-02376 Company, LLC; Lead es/prop65/notIces/2021- Root Powder 9 Walmart, Inc. -

Corporate Governance Report 30 June 2008
Corporate Governance Report 30 June 2008 Board of Directors Executive Board Contents Preliminary remarks 3 1. Board of Directors 4 1.1 Members of the Board of Directors 4 1.2. Professional background and other activities and functions 6 1.3 Cross-involvement 8 1.4 Internal organisational structure 9 2. Executive Board 12 2.1 Members of the Executive Board 12 2.2. Professional background and other activities and functions 14 General Organisation of Nestlé S.A. 15 Situation at 30 June 2008 © 2008, Nestlé S.A., Cham and Vevey (Switzerland) Concept: Nestlé S.A., Group Governance, Vevey (Switzerland) Design: Nestec Ltd., Corporate Identity and Design, Vevey (Switzerland) 2 Nestlé | Corporate Governance Report June 2008 Preliminary remarks Nestlé S.A. publishes a full Corporate Governance Report, including a separate Compensation Report, which forms an integral part of the annual Management Report. We therewith comply with the requirements of the SWX Swiss Exchange (SWX) and its Corporate Governance Directive. The present document is a partial update of the Nestlé Corporate Governance Report 2007, indicating changes occurred on the Board of Directors and the Executive Board up to 30 June 2008. The annual Management Report is available on-line as a PDF file at http://www.nestle.com in English, French and German. Copies can be ordered at: http://www.nestle.com/MediaCenter/Order. Contact for Media: Nestlé S.A. Corporate Media Relations Avenue Nestlé 55 CH - 1800 Vevey (Switzerland) tel. +41 (0)21 924 22 00 fax +41 (0)21 922 63 34 e-mail: [email protected] Contact for Investors: Nestlé S.A. -

Passover - Pesach April 2016 - Nissan 5776 2
1 PASSOVER - PESACH APRIL 2016 - NISSAN 5776 2 Good Design is Good Business Call us today to upgrad you e r marketing materials. L A U R E N M A N N D E S I G N 323.774.3225 • [email protected] WWW.LAURENMANNDESIGN.COM BUSINES S GRAPHICS AND PRINTING & CU STOM INV ITATIONS 3 TABLE OF CONTENTS cake, cereal made from wheat, oats Chometz on Pesach ...............................................................................3 or barley, cookies, farfel mix, some Kitniyos & Matzo Ashira ........................................................................3 licorice, pasta, pretzels, rolled oats, Bitul of Chometz .....................................................................................4 wheat gluten, wheat protein. Some Consumption of Chometz .................................................................... 5 have the same custom concerning beer and whiskey. Selling Of Chometz ................................................................................5 Chometz She’avar Alav Hapesach ......................................................5 Kashering Utensils .................................................................................6 STANDARDS OF KASHRUS Erev Pesach ..............................................................................................7 With over 1000 Kosher certifying Fast of the First Born ..............................................................................7 agencies and individuals competing Eating on Erev Pesach ...........................................................................7 -

Impact of Preoperative Endoscopic Ultrasound in Surgical Oncology
REVIEW Impact of preoperative endoscopic ultrasound in surgical oncology Endoscopic ultrasound (EUS) has a strong impact on the imaging and staging of solid tumors within or in close proximity of the upper GI tract. Technological developments during the last two decades have increased the image quality and allowed very detailed visualization of local tumor spread and lymph node affection. Current indications for EUS of the upper GI tract encompass the differentiation between benign and malignant lesions, the staging of esophageal, gastric and pancreatic cancer, and the procurement of a biopsy specimen through fine-needle aspiration. Various technical innovations during the past two decades have increased the diagnostic quality and have simultaneously strengthened the role of EUS in the clinical setting. This article will give a compressed summary on the current state of EUS and possible further technical developments. 1 KEYWORDS: 3D imaging elastosonography endoscopic ultrasound miniprobes Sascha S Chopra & oncologic surgery Michael Hünerbein† 1Department of General & Transplantation Surgery, Charité Campus Virchow-Clinic, Berlin, Conventional endoscopic ultrasound the so-called ‘miniprobes’ into the biliary system Germany Linear versus radial systems or the pancreatic duct in order to obtain high-res- †Author for correspondence: Department of Surgery & Surgical Endoscopic ultrasound (EUS) with flex- olution radial ultrasound images locally. Present Oncology, Helios Hospital Berlin, ible endoscopes is an important diagnostic and mini probes show a diameter of 2–3 mm and oper- 13122 Berlin, Germany Tel.: +49 309 417 1480 therapeutic tool, especially for the local staging ate with frequencies between 12 and 30 MHz. Fax: +49 309 417 1404 of gastrointestinal (GI) cancers, the differen- The main drawbacks of these devices are the lim- michael.huenerbein@ tiation between benign and malignant tumors, ited durability and the decreased depth of penetra- helios-kliniken.de and interventional procedures, such as biopsies tion (~2 cm). -

Immunoscintigraphy and Radioimmunotherapy in Cuba: Experiences with Labeled Monoclonal Antibodies for Cancer Diagnosis and Treatment (1993–2013)
Review Article Immunoscintigraphy and Radioimmunotherapy in Cuba: Experiences with Labeled Monoclonal Antibodies for Cancer Diagnosis and Treatment (1993–2013) Yamilé Peña MD PhD, Alejandro Perera PhD, Juan F. Batista MD ABSTRACT and therapeutic tools. The studies conducted demonstrated the good INTRODUCTION The availability of monoclonal antibodies in Cuba sensitivity and diagnostic precision of immunoscintigraphy for detect- has facilitated development and application of innovative techniques ing various types of tumors (head and neck, ovarian, colon, breast, (immunoscintigraphy and radioimmunotherapy) for cancer diagnosis lymphoma, brain). and treatment. Obtaining different radioimmune conjugates with radioactive isotopes OBJECTIVE Review immunoscintigraphy and radioimmunotherapy such as 99mTc and 188Re made it possible to administer radioimmuno- techniques and analyze their use in Cuba, based on the published lit- therapy to patients with several types of cancer (brain, lymphoma, erature. In this context, we describe the experience of Havana’s Clini- breast). The objective of 60% of the clinical trials was to determine cal Research Center with labeled monoclonal antibodies for cancer pharmacokinetics, internal dosimetry and adverse effects of mono- diagnosis and treatment during the period 1993–2013. clonal antibodies, as well as tumor response; there were few adverse effects, no damage to vital organs, and a positive tumor response in a EVIDENCE ACQUISITION Basic concepts concerning cancer and substantial percentage of patients. monoclonal antibodies were reviewed, as well as relevant inter- national and Cuban data. Forty-nine documents were reviewed, CONCLUSIONS Cuba has experience with production and radiola- among them 2 textbooks, 34 articles by Cuban authors and 13 by beling of monoclonal antibodies, which facilitates use of these agents. -
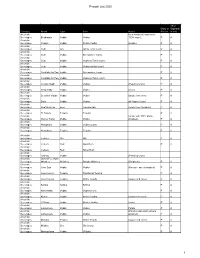
Pesach List 2020 1
Pesach List 2020 All or Dairy or Sephardi Category Brand Type Item Notes Pareve m only Alcoholic from Australia (made from Beverages Bushman's Vodka Vodka 100% cane). P A Alcoholic Beverages Chopin Vodka Potato Vodka (potato). P A Alcoholic Beverages Club Gin Gin & Tonic (corn). P A Alcoholic Beverages Club Vodka Screwdriver (corn). P A Alcoholic Beverages Club Vodka Vodka & Tonic (corn). P A Alcoholic Beverages Club Vodka Vodka Gimlet (corn). P A Alcoholic Beverages Cocktails for Two Vodka Screwdriver (corn). P A Alcoholic Beverages Cocktails for Two Vodka Vodka & Tonic (corn). P A Alcoholic Beverages Crystal Head Vodka Vodka (Peach & Corn) P A Alcoholic Beverages Deep Eddy Vodka Vodka (corn) P A Alcoholic Beverages Devotion Vodka Vodka Vodka (made from corn) P A Alcoholic Beverages Dixie Vodka Vodka all 4 types (corn) P A Alcoholic Beverages Dogfish Head Beer Tweason'ale (made from Sorghum) P A Alcoholic Beverages El Tosoro. Tequila Tequila P A Alcoholic (made with 100% Idaho Beverages Glacier Teton Vodka Vodka potatoes). P A Alcoholic Beverages Hampton's Vodka Vodka (Corn) P A Alcoholic Beverages Herradura. Tequila Tequila P A Alcoholic Beverages Iceberg Gin Gin P A Alcoholic Beverages Iceberg Rum Gold Rum P A Alcoholic Beverages Iceberg Rum Silver Rum Alcoholic Beverages Iceberg Vodka (Peach & Corn) Alcoholic James F.C. Hyde Beverages Whiskey Whiskey Sorgho Whiskey (Sorghum) P A Alcoholic Beverages Jinro Soju Vodka Vodka (Korean - sweet potatoes) P A Alcoholic Beverages Jose Cuervo Tequila Traditional Tequila P A Alcoholic Beverages Jose Cuervo Tequila White Tequila (agave and cane). P A Alcoholic Beverages Kahlua Kahlua Kahlua P A Alcoholic Beverages Kamchatka Vodka Vodka (corn). -

PHOENIX Chefswarehouse.Com BAKING and PASTRY TORTILLAS/WRAPS
PRODUCT CATALOG PHOENIX chefswarehouse.com BAKING AND PASTRY TORTILLAS/WRAPS......................8 BEVERAGES, HAVARTI.......................................19 CONDIMENTS BAKING MIXES ............................4 WRAPPERS..................................8 COFFEE AND TEA JACK CHEESE .............................19 AND JAMS BROWNIES ..................................10 MASCARPONE ...........................19 BAKING SUPPLIES .......................4 BAR MIXERS ................................15 CHUTNEY ....................................24 CAKES ASSORTED ......................10 MISCELLANEOUS........................19 COLORANTS ...............................4 CORDIAL ....................................15 GLAZES AND DEMI-GLAZES .......24 TARTS ...........................................10 MOUNTAIN STYLE........................19 CROISSANTS ...............................4 JUICE ...........................................15 KETCHUP .....................................24 COULIS........................................10 MOZZARELLA..............................20 DÉCOR ........................................4 MISCELLANEOUS BEVERAGE ....15 MAYO ..........................................24 PUREE..........................................10 MUENSTER...................................20 EXTRACTS ....................................4 NECTAR .......................................15 MUSTARD ....................................24 ZEST..............................................10 PARMESAN .................................20 FILLING ........................................4 -

Chapter 12 Monographs of 99Mtc Pharmaceuticals 12
Chapter 12 Monographs of 99mTc Pharmaceuticals 12 12.1 99mTc-Pertechnetate I. Zolle and P.O. Bremer Chemical name Chemical structure Sodium pertechnetate Sodium pertechnetate 99mTc injection (fission) (Ph. Eur.) Technetium Tc 99m pertechnetate injection (USP) 99m ± Pertechnetate anion ( TcO4) 99mTc(VII)-Na-pertechnetate Physical characteristics Commercial products Ec=140.5 keV (IT) 99Mo/99mTc generator: T1/2 =6.02 h GE Healthcare Bristol-Myers Squibb Mallinckrodt/Tyco Preparation Sodium pertechnetate 99mTc is eluted from an approved 99Mo/99mTc generator with ster- ile, isotonic saline. Generator systems differ; therefore, elution should be performed ac- cording to the manual provided by the manufacturer. Aseptic conditions have to be maintained throughout the operation, keeping the elution needle sterile. The total eluted activity and volume are recorded at the time of elution. The resulting 99mTc ac- tivity concentration depends on the elution volume. Sodium pertechnetate 99mTc is a clear, colorless solution for intravenous injection. The pH value is 4.0±8.0 (Ph. Eur.). Description of Eluate 99mTc eluate is described in the European Pharmacopeia in two specific monographs de- pending on the method of preparation of the parent radionuclide 99Mo, which is generally isolated from fission products (Monograph 124) (Council of Europe 2005a), or produced by neutron activation of metallic 98Mo-oxide (Monograph 283) (Council of Europe 2005b). Sodium pertechnetate 99mTc injection solution satisfies the general requirements of parenteral preparations stated in the European Pharmacopeia (Council of Europe 2004). The specific activity of 99mTc-pertechnetate is not stated in the Pharmacopeia; however, it is recommended that the eluate is obtained from a generator that is eluted regularly, 174 12.1 99mTc-Pertechnetate every 24 h.