Zinc-Responsive Dermatosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neonatal Dermatology Review
NEONATAL Advanced Desert DERMATOLOGY Dermatology Jennifer Peterson Kevin Svancara Jonathan Bellew DISCLOSURES No relevant financial relationships to disclose Off-label use of acitretin in ichthyoses will be discussed PHYSIOLOGIC Vernix caseosa . Creamy biofilm . Present at birth . Opsonizing, antibacterial, antifungal, antiparasitic activity Cutis marmorata . Reticular, blanchable vascular mottling on extremities > trunk/face . Response to cold . Disappears on re-warming . Associations (if persistent) . Down syndrome . Trisomy 18 . Cornelia de Lange syndrome PHYSIOLOGIC Milia . Hard palate – Bohn’s nodules . Oral mucosa – Epstein pearls . Associations . Bazex-Dupre-Christol syndrome (XLD) . BCCs, follicular atrophoderma, hypohidrosis, hypotrichosis . Rombo syndrome . BCCs, vermiculate atrophoderma, trichoepitheliomas . Oro-facial-digital syndrome (type 1, XLD) . Basal cell nevus (Gorlin) syndrome . Brooke-Spiegler syndrome . Pachyonychia congenita type II (Jackson-Lawler) . Atrichia with papular lesions . Down syndrome . Secondary . Porphyria cutanea tarda . Epidermolysis bullosa TRANSIENT, NON-INFECTIOUS Transient neonatal pustular melanosis . Birth . Pustules hyperpigmented macules with collarette of scale . Resolve within 4 weeks . Neutrophils Erythema toxicum neonatorum . Full term . 24-48 hours . Erythematous macules, papules, pustules, wheals . Eosinophils Neonatal acne (neonatal cephalic pustulosis) . First 30 days . Malassezia globosa & sympoidalis overgrowth TRANSIENT, NON-INFECTIOUS Miliaria . First weeks . Eccrine -

Association Between Vitamin D and Zinc Levels with Alopecia Areata Phenotypes at a Tertiary Care Center
Open Access Original Article DOI: 10.7759/cureus.14738 Association Between Vitamin D and Zinc Levels With Alopecia Areata Phenotypes at a Tertiary Care Center Saeed M. Alamoudi 1 , Siham M. Marghalani 2 , Rakan S. Alajmi 1 , Yara E. Aljefri 1 , Abdullah F. Alafif 1 1. Medicine, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, SAU 2. Dermatology, King Abdulaziz Medical City, Western Region, Jeddah, SAU Corresponding author: Saeed M. Alamoudi, [email protected] Abstract Objectives Alopecia areata (AA) is a common immune-mediated hair disorder that presents in different clinical patterns. This study aims to find the association between vitamin D and zinc levels with AA phenotypes, to determine the common comorbidities in AA patients, and to assess the influence of age, gender, and body mass index (BMI) on AA phenotypes. Methods This is a cross-sectional study conducted at King Abdulaziz Medical City (KAMC) in Jeddah, Saudi Arabia. Data were collected through retrospective chart review of the electronic medical record system (BestCare) and by utilizing a structured data collection sheet. Results A total of 177 patients were clinically diagnosed with AA with a mean age of 28.37 ± 12.68 years. The mean vitamin D level was 49.14 ± 29.09 nmol/L. Zinc levels were reported in only 22 patients, among which, only one patient had deficient levels. The mean zinc level was 9.8 ± 1.5 µmol/L. Patchy alopecia areata (60.45%) was the most common phenotype followed by universalis (9%) and totalis (7%). Hypothyroidism (11.8%) was the most prevalent comorbidity followed by atopic diseases (10.7%), then both diabetes and mood disorders (6.2%). -

A Review of Cutaneous Manifestations in Newborn Infants
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2017, 9[3]:1-8 [http://scholarsresearchlibrary.com/archive.html] ISSN 0975-5071 USA CODEN: DPLEB4 A Review of Cutaneous Manifestations in Newborn Infants Mohammad Ali Shakeri Hosseinabad* Ahvaz Jundishapur University of Medical Sciences, Resident of Dermatology, Ahvaz, Iran. *Corresponding author: Mohammad Ali Shakeri Hosseinabad, Ahvaz Jundishapur University of Medical Sciences, Resident of Dermatology, Ahvaz, Iran, Email: [email protected] _______________________________________________________ ABSTRACT Introduction: manifestations are very common in infants, and it can be a serious concern for parents, although most of them are benign and transient but some of them need further evaluation, accordingly knowledge about manifestations associated with infants can be an effective aid in the early diagnosis and treatment. The range of skin disorders is very wide. Timely examination of the skin in infants and control them is a good indicator to maintain the health of babies. Material and method: The required information through searching key words like: cutaneous manifestations, rashes, cutaneous infections, neonatal acne, early diagnosis, by Google Scholar, PubMed, Scopus, Magiran, Sid and Irandoc [from 1990 to 2016] was done Which some relevant articles were found and examined. Among 82 articles only 19 papers were quite relevant to cutaneous rashes. Findings: The first review of articles associated with this disease and infection and cutaneous rashes among infants and early diagnosis make a great help in timely treatment. Conclusion: one of the common cutaneous diseases is rashes, which treatment of bacterial or viral rashes is depends on its cause. Since that time of the disease is limited, in many patients and people with mild symptoms, do not need treatment. -

An Analysis of the Safety and Efficacy of Topical Zinc-Thymulin to Treat
y & Tran ap sp r la e n h t T a r t i i o a n H Hair : Therapy & Transplantation Vickers, Hair Ther Transplant 2017, 7:1 DOI: 10.4172/2167-0951.1000147 ISSN: 2167-0951 Research Article Open Access An Analysis of the Safety and Efficacy of Topical Zinc-Thymulin to treat Androgenetic Alopecia Vickers ER Pain Management and Research Centre, University of Sydney, Australia *Corresponding author: Vickers ER, Director of Clinical Stem Cells Pty Ltd., Australia, Tel: +61427711888; E-mail: [email protected] Received date: January 09, 2017; Accepted date: January 18, 2017; Published date: January 26, 2017 Copyright: © 2017 Vickers ER. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Objective: To assess the safety and efficacy of the metallopeptide zinc-thymulin (ZT) for treating androgenetic alopecia (AGA). Previous in vitro studies have described that different thymic peptides can both increase and decrease anagen (thymulin and thymosin beta-4, respectively). Zinc is an essential element and serum zinc deficiency can cause hair loss. Methods: Eighteen consecutive adult subjects were recruited, 17 males and 1 female, age range 35-90 years (mean 55.4, SD 13.3) with a diagnosis of AGA, Norwood classification 2-7, and hair loss duration range of 3-40 years (mean 15.8, SD 9.6). The trial duration for each subject ranged from 4-10 months. The test compound ZT was synthesized by standard Fmoc peptide protocols and administered in water based topical spray to the scalp. -
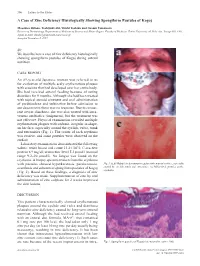
A Case of Zinc Deficiency Histologically Showing Spongiform Pustules of Kogoj
306 Letters to the Editor A Case of Zinc Deficiency Histologically Showing Spongiform Pustules of Kogoj Masahisa Shindo, Toshiyuki Aki, Yuichi Yoshida and Osamu Yamamoto Division of Dermatology, Department of Medicine of Sensory and Motor Organs, Faculty of Medicine, Tottori University, 86 Nishi-cho, Yonago 683-8503, Japan. E-mail: [email protected] Accepted November 5, 2007. Sir, We describe here a case of zinc deficiency histologically showing spongiform pustules of Kogoj during enteral nutrition. CASE REPORT An 89-year-old Japanese woman was referred to us for evaluation of multiple scaly erythematous plaques with erosions that had developed over her entire body. She had received enteral feeding because of eating disorders for 9 months. Although she had been treated with topical steroid ointment and oral administration of prednisolone and terbinafine before admission to our department, there was no response. Due to concur- rent severe diarrhoea, she was also treated with intra- venous antibiotics (imipenem), but the treatment was not effective. Physical examination revealed multiple erythematous plaques with oedema, irregular in shape, on her face, especially around the eyelids, vulva, trunk and extremities (Fig. 1). The centre of each erythema was erosive, and some pustules were observed on the surface. Laboratory examinations demonstrated the following values: white blood cell count 13.2 × 109/l; C-reactive protein 6.9 mg/dl; serum zinc level 5.2 μmol/l (normal range 9.2–20 μmol/l). No fungus was found on the erythema. A biopsy specimen taken from the erythema with pustules showed hyperkeratosis, parakeratosis, Fig. 1. -

UC Davis Dermatology Online Journal
UC Davis Dermatology Online Journal Title Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition Permalink https://escholarship.org/uc/item/2w1240vk Journal Dermatology Online Journal, 23(7) Authors Wu, Davina Fung, Maxwell A Kiuru, Maija et al. Publication Date 2017 DOI 10.5070/D3237035735 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Volume 23 Number 7 | July 2017 Dermatology Online Journal || Case Presentation DOJ 23 (7): 7 Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition Davina Wu1 MD PhD, Maxwell A Fung1 MD, Maija Kiuru1 MD PhD, Victoria R Sharon2 MD DTMH Affiliations:1 Department of Dermatology, University of California, Davis, California, 2Department of Dermatology, Hofstra Northwell School of Medicine, New Hyde Park, New York Corresponding Author: Victoria R. Sharon, MD, DTMH, 1991 Marcus Ave, Suite 300, New Hyde Park, NY 11040, USA, Email: vsharon@ northwell.edu Abstract This histologic pattern can also be seen in other conditions associated with nutritional deficiency Acquired zinc deficiency can develop as a consequence such as necrolytic acral erythema and pellagra. of poor nutritional intake or from dependence on total parenteral nutrition. Acquired zinc deficiency Variants in clinical and histopathologic presentation dermatitis classically manifests with erosions and of this dermatitis include vesicular and bullous forms scaly plaques in a periorificial and acral distribution. [3-5]. Histopathology associated with bullous, zinc We present a case of a woman on parenteral nutrition deficiency has been described to demonstrate intra- who presented with bullous acrodermatitis mimicking and subepidermal vesiculation and bullae [4]. -

Correlation Between the Severity and Type of Acne Lesions with Serum Zinc Levels in Patients with Acne Vulgaris
Hindawi Publishing Corporation BioMed Research International Volume 2014, Article ID 474108, 6 pages http://dx.doi.org/10.1155/2014/474108 Research Article Correlation between the Severity and Type of Acne Lesions with Serum Zinc Levels in Patients with Acne Vulgaris Majid Rostami Mogaddam,1 Nastaran Safavi Ardabili,2 Nasrollah Maleki,3 and Maedeh Soflaee1 1 Department of Dermatology, Imam Khomeini Hospital, Ardabil University of Medical Sciences, Ardabil 5618953141, Iran 2 Department of Midwifery, Islamic Azad University, Ardabil branch, Ardabil 5615731567, Iran 3 Department of Internal Medicine, Imam Khomeini Hospital, Ardabil University of Medical Sciences, Ardabil 5618953141, Iran Correspondence should be addressed to Nasrollah Maleki; [email protected] Received 6 February 2014; Accepted 14 July 2014; Published 24 July 2014 Academic Editor: Marian K. Malde Copyright © 2014 Majid Rostami Mogaddam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Acne vulgaris is the most common cutaneous disorder affecting adolescents and young adults. Some studies have reported an association between serum zinc levels and acne vulgaris. We aimed to evaluate the serum zinc level in patients with acne vulgaris and compare it with healthy controls. One hundred patients with acne vulgaris and 100 healthy controls were referred to our clinic. Acne severity was classified according to Global Acne Grading System (GAGS). Atomic absorption spectrophotometry was used to measure serum zinc levels. Mean serum level of zinc in acne patients and controls was 81.31 ± 17.63 g/dl and 82.63 ± 17.49 g/dl, respectively. -

Comparative Study of Serum Zinc Concentration In
Ranjbar et al. BMC Oral Health (2020) 20:296 https://doi.org/10.1186/s12903-020-01277-2 RESEARCH ARTICLE Open Access Comparative study of serum zinc concentration in recurrent herpes labialis patients and healthy individuals Zahra Ranjbar1, Maryam Zahed1* , Mohammad Ali Ranjbar2 and Zahra Shirmardan3 Abstract Background: Recurrent herpes labialis (RHL) is a common recurrent infective vesiculoulcerative disease. Topical and systemic administration of Zinc compounds has been indicated to have preventive and therapeutic efects. The pur- pose of this study was to evaluate the serum level of zinc in the patients with RHL and healthy individuals and also to investigate the correlation of this level with various parameters of the patient and disease course. Methods: This cross-sectional study was performed on 43 patients with a history of recurrent herpers labialis and 42 subjects without any previous experience of the lesion. Blood samples were taken, and serum zinc level was meas- ured using colorimetry (spectrophotometry) method. Chi-Square test was used to compare the qualitative relation- ships, and for comparing the quantitative relationships, independent T-test was used. To observe the relationshipof quantitative factors including serum zinc level, the number of relapses, and recovery rates, correlation test was taken. Results: The results show that, serum zinc level has no signifcant diference between healthy subjects and the patients (p > 0.05). Also, zinc level was not related to age and sex factors and frequency of relapse (p > 0.05). However surprisingly, there was a signifcant relationship between zinc level and recovery period in the RHL patients. The lower the serum zinc level, the higher the duration of recovery (p 0.009). -

Evaluation of Zinc, Vitamin B12, Folic Acid and Iron Levels and Thyroid
Ozuguz et al. J Dermatol Res Ther 2015, 1:1 ISSN: 2469-5750 Journal of DOI: 10.23937/2469-5750/1510008 Dermatology Research and Therapy Research Article: Open Access Evaluation of Zinc, Vitamin B12, Folic Acid and Iron Levels and Thyroid Functions in Patients with Chronic Telogen Effluvium Pinar Ozuguz1*, Seval Dogruk Kacar1, Ozlem Ekiz2 and Semsettin Karaca3 1 Check for Faculty of Medicine, Department of Dermatology, Afyon Kocatepe University, Turkey updates 2Faculty of Medicine, Department of Dermatology, Mustafa Kemal University, Turkey 3Department of Dermatology, Izmir Katip Celebi University, Turkey *Corresponding author: Pınar Ozuguz, Associate professor, Department of Dermatology, Afyon Kocatepe University, Turkey, Tel: 0905055210335, Fax: 0902722463300, E-mail: [email protected], [email protected] if persists more than 6 months. There may be no identifiable cause Abstract for TE. However, endocrine disorders, autoimmune aetiology, Background and Aim: Telogen effluvium (TE) is a hair disorder stressful events, nutritional disorders, localised and systemic skin characterized by abrupt onset, diffuse, self-limited and excessive diseases, intoxication, drugs, genetics and environmental factors shedding of club hairs. The purpose of this study was to investigate can be listed among many causes of hair loss [2,3]. If the hair goes serum zinc, vitamin B12 and folic acid levels as well as parameters of iron metabolism and thyroid functions in patients with chronic unwashed for more than 24 hours, usually more than 10% of the total telogen effluvium (CTE) and compare the results to those of the hair is easily extracted from any part of the scalp in the acute phase controls. of TE. -

Differential Diagnosis of Neonatal and Infantile Erythroderma
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Acta Dermatovenerol Croat 2007;15(3):178-190 REVIEW Differential Diagnosis of Neonatal and Infantile Erythroderma Lena Kotrulja1, Slobodna Murat-Sušić2, Karmela Husar2 1University Department of Dermatology and Venereology, Sestre milosrdnice University Hospital; 2University Department of Dermatology and Venereology, Zagreb University Hospital Center and School of Medicine, Zagreb, Croatia Corresponding author: SUMMARY Neonatal and infantile erythroderma is a diagnostic and Lena Kotrulja, MD, MS therapeutic challenge. Numerous underlying causes have been reported. Etiologic diagnosis of erythroderma is frequently difficult to University Department of Dermatology establish, and is usually delayed, due to the poor specificity of clinical and Venereology and histopathologic signs. Differential diagnosis of erythroderma is Sestre milosrdnice University Hospital a multi-step procedure that involves clinical assessment, knowledge of any relevant family history and certain laboratory investigations. Vinogradska 29 Immunodeficiency must be inspected in cases of severe erythroderma HR-10000 Zagreb with alopecia, failure to thrive, infectious complications, or evocative Croatia histologic findings. The prognosis is poor with a high mortality rate [email protected] in immunodeficiency disorders and severe chronic diseases such as Netherton’s syndrome. Received: June 14, 2007 KEY WORDS: erythroderma, neonatal, infantile, generalized Accepted: July 11, 2007 exfoliative dermatitis INTRODUCTION Erythroderma is defined as an inflammatory Neonatal and infantile erythroderma is a diag- skin disorder affecting total or near total body sur- nostic and therapeutic challenge. Erythrodermic face with erythema and/or moderate to extensive neonates and infants are frequently misdiagnosed scaling (1). It is a reaction pattern of the skin that with eczema and inappropriate topical steroid can complicate many underlying skin conditions at treatment can lead to Cushing syndrome. -

Hair's Zinc Level on Androgenic Alopecia
American Journal of Clinical and Experimental Medicine 2016; 4(5): 129-133 http://www.sciencepublishinggroup.com/j/ajcem doi: 10.11648/j.ajcem.20160405.13 ISSN: 2330-8125 (Print); ISSN: 2330-8133 (Online) Hair’s Zinc Level on Androgenic Alopecia Nurul Rumila Roem 1, Farida Tabri 1, Nurelly N. Waspodo 1, Ilhamjaya Patellongi 2, Agussalim Bukhari 3, Nursiah La Nafie 4 1Department of Dermatology and Venereology, Medical Faculty, Hasanuddin University, Makassar, Indonesia 2Department of Biostatistic, Faculty of Public Health, Hasanuddin University, Makassar, Indonesia 3Department of Clinical Nutrition, Medical Faculty, Hasanuddin University, Makassar, Indonesia 4Department of Chemical, Mathematics and Sciences Faculty, Hasanuddin University, Makassar, Indonesia Email address: [email protected] (N. R. Roem) To cite this article: Nurul Rumila Roem, Farida Tabri, Nurelly N. Waspodo, Ilhamjaya Patellongi, Agussalim Bukhari, Nursiah La Nafie. Hair’s Zinc Level on Androgenic Alopecia. American Journal of Clinical and Experimental Medicine. Vol. 4, No. 5, 2016, pp. 129-133. doi: 10.11648/j.ajcem.20160405.13 Received : July 19, 2016; Accepted : July 30, 2016; Published : August 25, 2016 Abstract: Androgenic alopecia is characterized by progressive loss of hair from the scalp. This research aimed to determine the hair and blood zinc levels in men with androgenic alopecia. The research was conducted in the Departement of Dermatovenereology of Dr. Wahidin Sudirohusodo Hospital, Makassar and the Center for Health Laboratory, Makassar, using the observational research method. The samples comprised 21 males with androgenic alopecia and 11 control samples without androgenic alopecia. The hair and blood of the samples were analyzed using the atomic absorption spectrophotometer receipts. The results showed that there was significant difference (p <0.05) between the levels of androgenic alopecia hair zinc and control. -

Trace Element Levels in Alopecia Areata
Original TTracerace eelementlement llevelsevels iinn aalopecialopecia aareatareata Article YYasmeenasmeen J.J. BBhat,hat, SSheikhheikh MManzoor,anzoor, AA.. RR.. KKhanhan1, SSeemaeema QQayoomayoom ABSTRACT Departments of Dermatology, STD and Leprosy, Background: Alopecia areata (AA) is a recurrent, nonscarring type of hair loss considered to 1Biochemistry and SKIMS be an autoimmune process. Though its etiopathology is not fully understood, there are claims Medical College Hospital, that imbalance of trace elements may trigger the onset of AA. Aim: The aim of the present Bemina, Srinagar, India study was to assess the levels of zinc, copper, and magnesium in the serum of AA patients. AAddressddress forfor ccorrespondence:orrespondence: Methods: Fifty AA patients (34 men and 16 women), and Þ fty age and sex matched healthy Dr. Yasmeen J. Bhat, control subjects were studied. Samples were analyzed using atomic absorption spectrometric Department of Dermatology, methods. Results: Serum zinc levels were signiÞ cantly decreased (P < 0.05) in AA patients STD and Leprosy, SKIMS, whose disease was extensive, prolonged, and resistant to treatment, whereas serum copper MCH, Srinagar, India. and magnesium levels showed insigniÞ cant rise compared to controls. Conclusion: We E-mail: yasmeen_bhat2001@ yahoo.co.in conclude that copper and magnesium levels are not altered in AA, but the decreased zinc levels found in our study may merit further investigation of the relationship. Key words: Alopecia areata, Copper, Magnesium, Zinc IINTRODUCTIONNTRODUCTION Clearance was obtained from the institutional ethical committee. Detailed history was recorded and clinical Alopecia areata (AA) is a recurrent, nonscarring type examination performed. A proper history in relation of hair loss that can affect any hair bearing area.