Redalyc.Spatial, Temporal and Size Distribution of Particulate Matter And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHINIOT, PUNJAB 1 MINI DISTRICT PROFILE for RAPID NEEDS ASSESSMENT September 15Th, 2014
RAIN/FLOODS 2014: CHINIOT, PUNJAB 1 MINI DISTRICT PROFILE FOR RAPID NEEDS ASSESSMENT September 15th, 2014 Rains/Floods 2014: Chiniot District Profile September 2014 iMMAP-USAID District at a Glance Administrative DivisionRajanpur - Reference Map Police Stations Attribute Value District/tehsil Knungo Patwar Number of Mouzas Police Station Phone No. Population (2013 est) 1,180,200 Circles/ Circles/ Male 612,924 (52%) Supervisory Tapas Total Rural Urban Partly Forest Un- SDPO City Circle 0476-332930 Tapas urban populated Female 567,276 (48%) City 0476-331520 DISTRICT 13 115 361 347 3 4 1 6 Rural 1,465,614 (78%) Source: Punjab Mouza Statistics 2008 Sadar 0476-331540 Urban 403,807 (22%) Rajoa 0346-5833304 Tehsils 3 Road Network Infrastructure UC 45 District Route Length SDPO Chanab Nagar Circle 0476-214890 Revenue Villages 361 Chiniot to Faislabad Narwala road 44 Km Chanab Nagar 0476-213175 Chiniot to Jhang Jhang- Chiniot road 123 Km Area (Sq km) 2,610.28 Police Post Chanab Nagar 0476-213176 Chiniot to Sargodha Faisalabad road 77.3 Km Registered Voters (ECP) 587,962 Chiniot to Hafizabad Lahore road 171 Km Lalian 0476-610210 Literacy Rate 10+ (PSLM 2010-11) 47% Chiniot-Lahore Lahore - Islamabad Motorway/AH1 161 KM Barrana 0300-8644435 Source: Google Maps Departmental Focal Points Kandiwal 0321-6000844 Entry-Exit Points SDPO Bhawana Circle 0476-201515 Designation Contacts 1 Lalian 4 Aminpur Commissioner, Faisalabad Division 041-9201701, 041-9201702 2 Jaisal 5 Chak 223 Bhawana 0476-201040 DCO, Chiniot 047-6330071 EDO(H) 0476-330106 3 Muhammad -
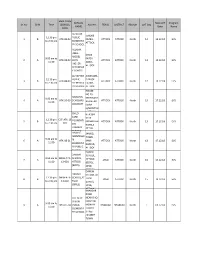
EMIS CODE SCHOOL New QAT Program Sr
EMIS CODE SCHOOL New QAT Program Sr. No Shift Time /SCHOOL Address TEHSIL DISTRICT REGION QAT Day NAME Date Name CODE AL NOOR DHOKE 12.30 pm PUBLIC 1 B ATK-08-02 FAREH, ATTOCK ATTOCK North 12 15.12.18 EVS to 2.30 pm ELEMENTA ATTOCK RY SCHOOL ALLAMA IQBAL DHOK MODEL 9.00 am to FATEH 2 A ATK-08-03 BOYS ATTOCK ATTOCK North 12 15.12.18 EVS 11.00 ABAD, HIGHER ATTOCK SECONDAR Y SCHOOL AL TAYYAB AWANABA 12.30 pm PUBLIC D DHOK 3 B ATK-08-04 ATTOCK ATTOCK North 12 15.12.18 EVS to 2.30 pm ELEMENTA FATAH, RY SCHOOL ATTOCK HOUSE NO.20 MUSLIMS 9.00 am to MOH.KARB 4 A ATK-10-05 SCHOLARS ATTOCK ATTOCK North 13 17.12.18 EVS 11.00 ALA NEAR ACADEMY GORA QABRISTAN , ATTOCK CHILD H. # 564 CARE CHHE 12.30 pm CCF-ATK-13- 5 B FOUNDATI MOHALLAH ATTOCK ATTOCK North 13 17.12.18 EVS to 2.30 pm 001 ON KARBLA PRIMARY ATTOK SCHOOL, HASNAT JAMEEL MONTESSO TOWN 9.00 am to 6 A ATK-08-01 RI MARI ATTOCK ATTOCK North 13 17.12.18 EVS 11.00 ELEMENTA KANJUR, RY PUBLIC ATTOCK SCHOOL DANISH DANISH SCHOOL, 9.00 am to DNSH-ATK- SCHOOL, 7 A ATTOCK JAND ATTOCK North 15 19.12.18 EVS 11.00 13-001 ATTOCK (BOYS), (BOYS) JAND, ATTOCK DANISH DANISH SCHOOL,AT 12.30 pm DNSH-ATK- SCHOOL,AT 8 B TOCK JAND ATTOCK North 15 19.12.18 EVS to 2.30 pm 13-002 TOCK (GIRLS), (GIRLS) JAND, ATTOCK KHANSAR ROAD THE NEW NEAR DHQ VISION HOSPITAL 9.00 am to 9 A BHK-07-04 PUBLIC MOBILIK BHAKKAR BHAKKAR North 1 03.12.18 EVS 11.00 ELEMENTA TOWER RY SCHOOL STREET ,NAZEER TOWN BAKHAR MABTALE- MAKTAB-E- DANISH DANISH 12.30 pm PUBLIC 10 B BHK-07-03 PUBLIC BHAKKAR BHAKKAR North 1 03.12.18 EVS to 2.30 pm SCHOOL SCHOOL ALAMABAD BHAKKAR , BHAKKAR QADIR FOUNDATI HAIDERAB ON AD THAL, 9.00 am to QDRF-BHK- TEHSIL 11 A ENGLISH MANKERA BHAKKAR North 6 08.12.18 EVS 11.00 15-001 MEDIUM MANAKERA PUBLIC , DISTRICT PRIMARY BHAKKAR. -

1. Short Title and Commencement. 2. Definitions. 3. Application of the Act
An Act to provide for the levy and collection of tax on certain lands in the Punjab Preamble. WHEREAS it is expedient to provide for the levy and collection of improvement tax on certain lands in the Punjab: It is hereby enacted as follows:- 1. Short title and commencement. 1. Short title and commencement. (1) This Act may be called the Punjab Lands Improvement Tax Act, 1975. (2) It shall come into force at once. 2. Definitions. 2. Definitions. (1) In this Act, unless there is anything repugnant in the subject or context,- (a) and means the land assessed to land revenue; (b) ocal Area means the area specified in the Schedule; (c) chedule means the Schedule appended to this Act; and (d) ax means the tax levied under this Act. (2) The words and expression used but not defined shall bear the same meaning as they bear in the West Pakistan[2] Land Revenue Act, 1967. 3. Application of the Act. 3. Application of the Act. This Act shall apply to the lands situated in the local areas specified in the Schedule. 4. Tax on lands in the local areas. 4. Tax on lands in the local areas. There shall be levied and collected tax from the owners of the lands situated in the local areas at the rate of Rs. 3.75 per acre per annum for a period of 12 years. pd4ml evaluation copy. visit http://pd4ml.com 5. Levy, collection and recovery of tax. 5. Levy, collection and recovery of tax. Tax shall be levied and collected in such manner as may be prescribed and the arrears of it may be recovered as if it were arrears of land revenue. -

Workshop List
Motor Claims Department Panel List of Workshops SR. CITY NAME TYPE WORKSHOP NAME ADDRESS 1 ABBOTTABAD 3S HONDA ABBOT (PRIVATE) LIMITED HONDA ABBOT, MAIN MANSEHRA ROAD, KALAPUR, PAKISTAN 2 AZAD KASHMIR 3S TOYOTA AZAD MOTORS MIAN MUHAMMAD ROAD MIRPUR 3 BAHAWALNAGAR 3S SUZUKI BAHAWALNAGAR MOTORS 1-KM, ARIF WALA BYPASS ROAD, 4 BAHAWALPUR NON 3S AUTO POINT AUTOMOTIVE MULTAN ROAD, NEAR SAFARI GARDEN, BAHAWALPUR 5 BAHAWALPUR 3S FAW BAHAWALPUR MOTORS MULTAN ROAD, NEAR SUTLEJ BRIDGE, OPP HUMERA HOTEL 6 BAHAWALPUR NON 3S LAHORE MOTOR WORKSHOP MULTANI GATE TIBYYA COLLEGE 7 BAHAWALPUR NON 3S LAHORE MOTORS WORKSHOP. MULTANI GATE TIBYYA COLLEGE BAHAWALPUR 8 BAHAWALPUR 3S SUZUKI BAHAWALPUR MOTORS OPP ZAKHEERA, AHMADPUR ROAD, BAHAWALPUR 9 BAHAWALPUR 3S TOYOTA BAHAWALPUR MOTORS (PVT) LTD KLP ROAD, NEAR KARACHI MORR BAHAWALPUR 10 CHUNIAN 3S SUZUKI CHUNIAN MOTORS MAIN CHANGA MANGA ROAD MIAN WALA GHAT, CHUNIAN 11 DERA GHAZI KHAN 3S HONDA HI-SUN MUSTAFA CHOWK MULTAN ROAD DG KHAN, 12 DERA GHAZI KHAN 3S SUZUKI D.G. KHAN MOTORS MAIN MULTAN ROAD, DERA GHAZI KHAN 13 DERA GHAZI KHAN 3S TOYOTA D.G. KHAN MOTORS INDUS HIGHWAY 7KM, JAMPUR ROAD, DERA GHAZI KHAN. 14 FAISALABAD NON 3S ABDULLAH AUTO WORKSHOP COMPANY BAGH ROAD, NEW CIVIL LINE, GENERAL BUS STAND FAISALABAD ABDULLAH PUR, NEAR NATIONAL BANK, ASHRAF DENTAL CLINIC, 15 FAISALABAD NON 3S ADNAN AUTO WORKSHOP FAISALABAD 16 FAISALABAD NON 3S AHMAD MOTOR WORKSHOP JARANWALA ROAD NEAR PETROL PUMP FAISALABAD 17 FAISALABAD 3S ASGHAR & SONS PVT LTD EAST CANAL ROAD, NEAR KASHMIR PULL FAISALABAD 18 FAISALABAD NON 3S AUTO -

Village List of Multan Division , Pakistan
Cel'.Us 51·No. 30B (I) M.lnt.6-19 300 CENSUS OF PAKISTAN, 1951 VILLAGE LIST PUNJAB Multan Division OFFICE Of THE PROVINCIAL · .. ·l),ITENDENT CENSUS, J~ 1952 ,~ :{< 'AND BAHAWALPUR, P,IC1!iR.. 10 , , FOREWOf~D This Village Ust has been prepared from the material collected in con nection with the Census of Pakistan, 1951. The object of the List is to present useful information about our villages. It was considered that in a predominantly rural country like Pakistan, reliable village statistics should be available and it is hoped that the Village List will form the basis for the continued collection of such statistics. A summary table of the totals for each tehsil showinz its area to the nearest square mile, and its population and the number of houses to the nearest hundred is given on page I together with the page number on which each tehsil begins. The general village table, which has been compiled district-wise and arranged tehsil-wise, appears on page 3 et seq. Within each tehsll th~ Revenue Kanungo ho/qas are shown according to their order in the census records. The Village in which the Revenue Kanungo usually resides is printed in bold type at the beginning of each Kanungo halqa and the remaining villages comprising the halqas, are shown thereunder in the order of their revenue hadbast numbers, which are given in column a. Rakhs (tree plantations) and other similar area,. even where they are allotted separate revenue hadbast nurY'lbcrs have not been shown as they were not reported in the Charge and Household summaries, to be inhabited. -

MDP-Sialkot-17Th Sep 2014
RAIN/FLOODS 2014: SIALKOT, PUNJAB 1 MINI DISTRICT PROFILE FOR RAPID NEEDS ASSESSMENT September 17th, 2014 Rains/Floods 2014: Sialkot District Profile September 2014 iMMAP-USAID District at a Glance Administrative DivisionR ajanpur - Reference Map Police Stations Attribute Value District/tehsil Knungo Patwar Number of Mouzas Sr. No. Police Station Phone No. Population (2014 est) 4,017,821 Circles/ Circles/ 1 SDPO City (Circle) 0092-52-9250346 Male 2,061,052 (51%) Supervisory Tapas Total Rural Urban Partly Forest Un- 2 Kotwali 0092-52-9250341-2 Tapas urban populated Female 1,956,769 (49%) 3 Civil Lines 0092-52-9250331-3 DISTRICT 22 302 1578 1401 35 30 11 101 Rural 2,965,481 (74%) 4 Cantt 0092-52-9250343-4 SIALKOT 7 98 576 479 22 13 11 51 Urban 1,052,340 (26%) TEHSIL 5 Rang Pura 0092-52-4583001 Tehsils 4 PASROOR 7 99 597 542 10 11 34 6 Neka Pura 0092-52-4592760 UC 129 DASKA 5 67 250 238 2 4 6 7 Haji Pura 0092-52-3553613 Revenue Villages 1,431 SAMBRIAL 3 38 155 142 1 2 10 8 Police Post Suchait Garh (P.S. Cantt) 0092-52-3206111 Source: Punjab Mouza Statistics 2008 Area (Sq km) 3,061 9 SDPO Sadar (Circle) 0092-52-3251111 Registered Voters (ECP) 1,061,843 10 Sadar Sialkot 0092-52-3542969 Road Network Infrastructure Literacy Rate 10+ (PSLM 2010-11) 66% District Route Length 11 Uggoki 0092-52-3570484 Sialkot to Narowal Pasrur road 53 Km 12 Murad Pur 0092-52-3562450 Departmental Focal Points Sialkot to Gujranwala Gujranwala road 45 Km 13 Kotli Loharan 0092-52-3533133 Title Phone Fax/Email Sialkot to Wazirabad Wazirabad road 46 Km 14 Kotli Said -

Spatial, Temporal and Size Distribution of Particulate Matter and Its Chemical Constituents in Faisalabad, Pakistan
UC Davis UC Davis Previously Published Works Title Spatial, temporal and size distribution of particulate matter and its chemical constituents in Faisalabad, Pakistan Permalink https://escholarship.org/uc/item/55x938xs Journal Atmosfera, 28(2) ISSN 0187-6236 Authors Javed, W Wexler, AS Murtaza, G et al. Publication Date 2015 DOI 10.1016/s0187-6236(15)30003-5 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Atmósfera 28(2), 99-116 (2015) Spatial, temporal and size distribution of particulate matter and its chemical constituents in Faisalabad, Pakistan WASIM JAVED Mechanical Engineering, Texas A&M University at Qatar, Doha, Qatar; Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad, Pakistan Corresponding author; e-mail: [email protected] ANTHONY S. WEXLER Air Quality Research Center, University of California-Davis, CA, USA GHULAM MURTAZA and HAMAAD R. AHMAD Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad, Pakistan SHAHZAD M. A. BASRA Department of Crop Physiology, University of Agriculture, Faisalabad-38040, Pakistan Received May 25, 2014; accepted February 6, 2015 RESUMEN Se investigaron las variaciones espaciales y temporales de aerosoles de material particulado (PM, por sus siglas en inglés) en Faisalabad, Pakistán, de junio de 2012 a abril de 2013 para conocer la distribución de componentes químicos y fracciones granulométricas en todas las estaciones del año. Se controlaron cuatro fracciones de PM (partículas -

Islamic Banking Branches ATM Locations
Islamic Banking Branches ATM Locations Branch Code Branch Name Branch Address City 3006 IBB-Leads Centre Leads Centre Branch, Shop # 4, 4A & 4B, Leads Centre, Main Boulevard, Gulberg-111, Lahore. Lahore 3025 IBB-Allama Iqbal Town 20 Gulshan Block Allama Iqbal Town Lahore Lahore 3037 IBB RAIWIND ROAD LAHORE 131/178, Bohbatein Chowk, Raiwind Road, Lahore Lahore 3040 IBB Exop Centre Plot No 590 Block H 3 Opposite Expo Centre M.A Johar Town Lahore Lahore 3051 IBB-Bahria Town IBB Plot No . 5/A sector C Bahria Town Laore. Lahore 3054 IBB KASUR BRANCH BIV -9R-220/A Outside Lahori Gate Railway Road Kasur Kasur 3067 IBB FEROZPUR ROAD MOHALLAH MAKHDOOM ABAD CHUNGI AMR SIDHU MAIN FEROZPUR ROAD LAHORE Lahore 3073 IBB ILLAHABAD Main Kasur Road Illahabad Kasur 3089 IBB Kot Radha Kishan Khewat no. 298,Khatooni # 403 to 418 near Ghala Kot Radha Kishan dist.Kasur Kot Radha Kishan 3116 IBB Phool Nagar Khewat # 230 Khatooni # 399 Salim Khata 81- Canal Main Bazar Phool Naagr Patoki 3117 IBB BUND ROAD Building No.SWVII-23-C-S-68, Nawan Sanda Main Band Road Lahore Lahore 3127 IBB-Lake City Plot # 14, Commercial Block # 36, Lake City Br.3127. Raiwind Road Lahore. Lahore 3130 IBB-Bahria Ochard Plot No.19, Eastern Block, Bahria Orchards,Main Raiwand Road, Lahore. Lahore 3132 IBB-MULL CHOWK LAHORE AL KHAN PLAZA NEAR SUNDER INDUSTRIAL ESTATE GATE NO 1 MULL CHOWK LAHORE Lahore 3139 IBB-Kahna Nau Property unit No. S86R-1968, Situated at Mouza Gajumata Tehsil Model Town Lahore Lahore Plot No. 5 Block-A Izmir Town (Pakistan Expatriates Co-operative Housing Society Izmir Ltd.) Main Canal Bank Road, 3156 IBB Izmir Town Lahore Lahore Khasra No.3432, Khewat No.624, Khatoni No.804 to 836,Opposite to EME Housing Society, Shahpur Kanjra,17 KM 3160 IBB-Shahpur Kanjra Lahore Multan Road,tehsil and district Lahore. -

Pakistan Hosiery Manufacturers & Exporters Association
PAKISTAN HOSIERY MANUFACTURERS & EXPORTERS ASSOCIATION PROVISIONAL LIST OF VOTERS - KARACHI ELECTION 2014-15 NAME OF SL MNO NAME OF COMPANY ADDRESS TEL NO. FAX NO. EMAIL STN & NTN REPRESENTATIVE PLOT NO. 1-K-18,NAZIMABAD MR. ABDUL QADIR 36902713- 36902716- kamani786@cyber. STN: 11-00-6200-036-64 1 0218 2-U INTERNATIONAL NO. 1, NEAR NAYAB CINEMA, KAMANI 4/36609855 36621006 net.pk NTN: 1426721-7 KARACHI. MR. JAWAID SULTAN A & J APPAREL (PVT) LA-6/A, BLOCK-22,FEDERAL "B" 36342521- aitazaz@ajapparel. STN: 11-00-1190-016-46 2 0532 36313684 JAPANWALA LTD. AREA, KARACHI. 36806540 com NTN: 0704117-9 24/1, SECTOR 6-A, NORTH MR. MUHAMMAD 36974878- 36908625- [email protected]. STN: 11-00-1190-178-46 3 0870 A. ESSAK & SONS KARACHI INDUSTRIAL AREA, YOUSUF ISMAIL 9/36956280 35206348 pk NTN: 0670673-8 NEAR SABA CINEMA, KARACHI. D-264, K.D.A. SCHEME # 35077506- 35057907- [email protected] STN: 12-00-5212-025-64 4 0598 MR. ALTAF MAJEED A. MAJEED & SONS 1,BEHIND TIMES MEDICOS 34860600-2 34122222 m NTN: 1162019-6 (STADIUM RD) KARACHI. PLOT NO. 8/5-1, FIRST FLOOR, MR. MUHAMMAD 36963001- [email protected] STN: 17-12-9999-400-37 5 1202 A.A. LACE WORKS SECTOR 12-C, NORTH KARACHI 36963001 AAMIR LARI 36951698- om NTN: 2249889-3 IND. AREA, KARACHI PLOT NO. DP-36, SECTOR 12- 38125838- 38045915- [email protected] STN: 17-12-5900-436-37 6 0944 MR. FAHEEM AKHTER A.F. EXPORT D,NORTH KARACHI IND. AREA, 36962733 36962735 om NTN: 2541424-7 KARACHI. -
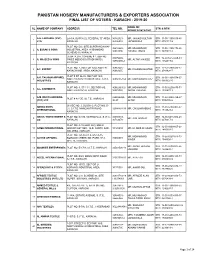
Final List of Voters - Karachi - 2019-20
PAKISTAN HOSIERY MANUFACTURERS & EXPORTERS ASSOCIATION FINAL LIST OF VOTERS - KARACHI - 2019-20 NAME OF SL NAME OF COMPANY ADDRESS TEL NO. STN & NTN REPRESENTATIVE A & J APPAREL (PVT) LA-6/A, BLOCK-22, FEDERAL "B" AREA, 36342521- MR. JAWAID SULTAN STN: 11-00-1190-016-46 1 LTD. KARACHI. 36806540 JAPANWALA NTN: 0704117-9 PLOT NO. D/3, SITE SUPER HIGHWAY 36412228- MR. MUHAMMAD STN: 11-00-1190-178-46 2 A. ESSAK & SONS INDUSTRIAL AREA, AHSANABAD 36881306 YOUSUF ISMAIL NTN: 0670673-8 SCHEME-33, KARACHI. D-264, K.D.A. SCHEME # 1, BEHIND 35075065- STN: 12-00-5212-025-64 3 A. MAJEED & SONS TIMES MEDICOS (STADIUM RD) MR. ALTAF MAJEED 34860600-2 NTN: 1162019-6 KARACHI. PLOT NO. 3, SECTOR 12/D, NORTH 36963225- STN: 17-12-5900-436-37 4 A.F. EXPORT MR. FAHEEM AKHTER KARACHI IND. AREA, KARACHI. 36963255 NTN: 2541424-7 PLOT # DP 84-85, SECTOR 12/C, A.K. FASHION APPAREL STN: 11-00-1190-074-37 5 NORTH KARACHI INDUSTRIAL AREA, 36950357-58 MR. MOHAMMAD RIAZ INDUSTRIES NTN: 0669324-5 KARACHI PLOT NO. 9, ST-13/1, SECTOR 6-B, 36962361-2 / MR. MOHAMMAD STN: 11-00-6200-875-73 6 A.L. GARMENTS NORTH KARACHI, KARACHI. 36978050 IMRAN LAKHANI NTN: 1532475-3 A.M. MERCHANDISING 325599095- MR. MUHAMMAD STN: 11-02-9999-395-37 7 PLOT # A-15/E, S.I.T.E., KARACHI. (PVT) LTD 96-97 ALTAF NTN: 3096186-6 SHADE NO. 2, BLOCK-2, PLOT NO. D- ABBAS KNITS STN: 11-00-6200-907-82 8 21, S.I.T.E. -

Punjab Education Foundation
Punjab Education Foundation TDP Training Batch-1 Clusters ATK-TDP-6 Progam EVS Training TDP District ATTOCK Tehsil ATTOCK Venue School Code ATK-10-05 Venue School Name MUSLIMS SCHOLARS ACADEMY Venue School Owner MALIK JAVED IQBAL Venue School Owner Contact 03445224028 School Owner Owner No. Of SR# School Name School Address Program Code Name Contact Teachers MALIK ATK-10- HOUSE NO.20 MOH.KARBALA 1 MUSLIMS SCHOLARS ACADEMY EVS JAVED 03445224028 5 05 NEAR GORA QABRISTAN IQBAL CCF- CHILD CARE FOUNDATION PRIMARY 2 ATK-13- SCHOOL,H. # 564 CHHE MOHALLAH KARBLA SODHRA EVS CCF 03059586264 5 001 ATTOK 9-ATK- MOHALLAH MASJID USMANIA, ZULFIQAR 3 Modern Public Elementary School FAS 03009844500 5 0006 SHINBAGH KHURD AHMAD ATK-08- HASNAT MONTESSORI ELEMENTARY RAFIA 4 JAMEEL TOWN MARI KANJUR EVS 03135059261 5 01 PUBLIC GULNAZ MOHALLAH SHAH FAISAL 9-ATK- Mohammad 5 Green Land Elementary School ABAD MAARI ROAD ATTOCK FAS 03445042703 5 0004 Sharif CITY BWN-TDP-17 Progam FAS Training TDP District BAHAWALNAGAR Tehsil BAHAWALNAGAR Venue School Code 220 Venue School Name IDEAL PUBLIC SCHOOL Venue School Owner MUHAMMAD AZAM Venue School Owner Contact 03347037623 School Owner No. Of SR# School Name School Address Program Owner Name Code Contact Teachers MUHAMMAD 1 220 IDEAL PUBLIC SCHOOL QURAISH COLONY BAHAWAL NAGAR FAS 03347037623 5 AZAM AL.QURAISH BRILLIANT QURAISH COLONY,NEAR RAILWAR MATEEN UR 2 209 FAS 03017689357 3 PUBLIC SCHOOL CROSSING.BAHAWALNAGAR REHMAN HI CALIBRE FOUNDATION CH. 3 219 353 JINNAH COLONY FAS 03347020820 5 SCHOOL ZULFIQAR ALI FAISAL PUBLIC HIGH KHALID 4 55 FAISAL COLONY BAHAWAL NAGAR FAS 03336337749 3 SCHOOL MAHMOOD MUHAMMAD PIONEER PUBLIC MIDDLE MUBARIK GATE TAKIA MOHSIN SHAH ROAD 5 689 FAS YASMIN 03347019352 5 SCHOOL SADAR DIN TOWN BAHAWAL NAGAR SABRI SAYYED-E-NA- 6 236 SADDIQUE-E-AKBAR MUSLIM COLONY JAIL ROAD BAHAWALNAGAR FAS M. -
Updated MDP-Chiniot
RAIN/FLOODS 2014: CHINIOT, PUNJAB 1 MINI DISTRICT PROFILE FOR RAPID NEEDS ASSESSMENT September 17th, 2014 Rains/Floods 2014: Chiniot District Profile September 2014 iMMAP-USAID District at a Glance Administrative Division Rajanpur - Reference Map Police Stations Attribute Value District/tehsil Knungo Patwar Number of Mouzas Police Station Phone No. Population (2013 est) 1,180,200 Circles/ Circles/ Male 612,924 (52%) Supervisory Tapas Total Rural Urban Partly Forest Un- SDPO City Circle 0476-332930 Tapas urban populated Female 567,276 (48%) City 0476-331520 DISTRICT 13 115 361 347 3 4 1 6 Rural 1,465,614 (78%) Source: Punjab Mouza Statistics 2008 Sadar 0476-331540 Urban 403,807 (22%) Rajoa 0346-5833304 Tehsils 3 Road Network Infrastructure UC 45 District Route Length SDPO Chanab Nagar Circle 0476-214890 Revenue Villages 361 Chiniot to Faislabad Narwala road 44 Km Chanab Nagar 0476-213175 Chiniot to Jhang Jhang- Chiniot road 123 Km Area (Sq km) 2,610.28 Police Post Chanab Nagar 0476-213176 Chiniot to Sargodha Faisalabad road 77.3 Km Registered Voters (ECP) 587,962 Chiniot to Hafizabad Lahore road 171 Km Lalian 0476-610210 Literacy Rate 10+ (PSLM 2010-11) 47% Chiniot-Lahore Lahore - Islamabad Motorway/AH1 161 KM Barrana 0300-8644435 Source: Google Maps Departmental Focal Points Kandiwal 0321-6000844 Entry-Exit Points SDPO Bhawana Circle 0476-201515 Designation Contacts 1 Lalian 4 Aminpur Commissioner, Faisalabad Division 041-9201701, 041-9201702 2 Jaisal 5 Chak 223 Bhawana 0476-201040 DCO, Chiniot 047-6330071 EDO(H) 0476-330106 3 Muhammad