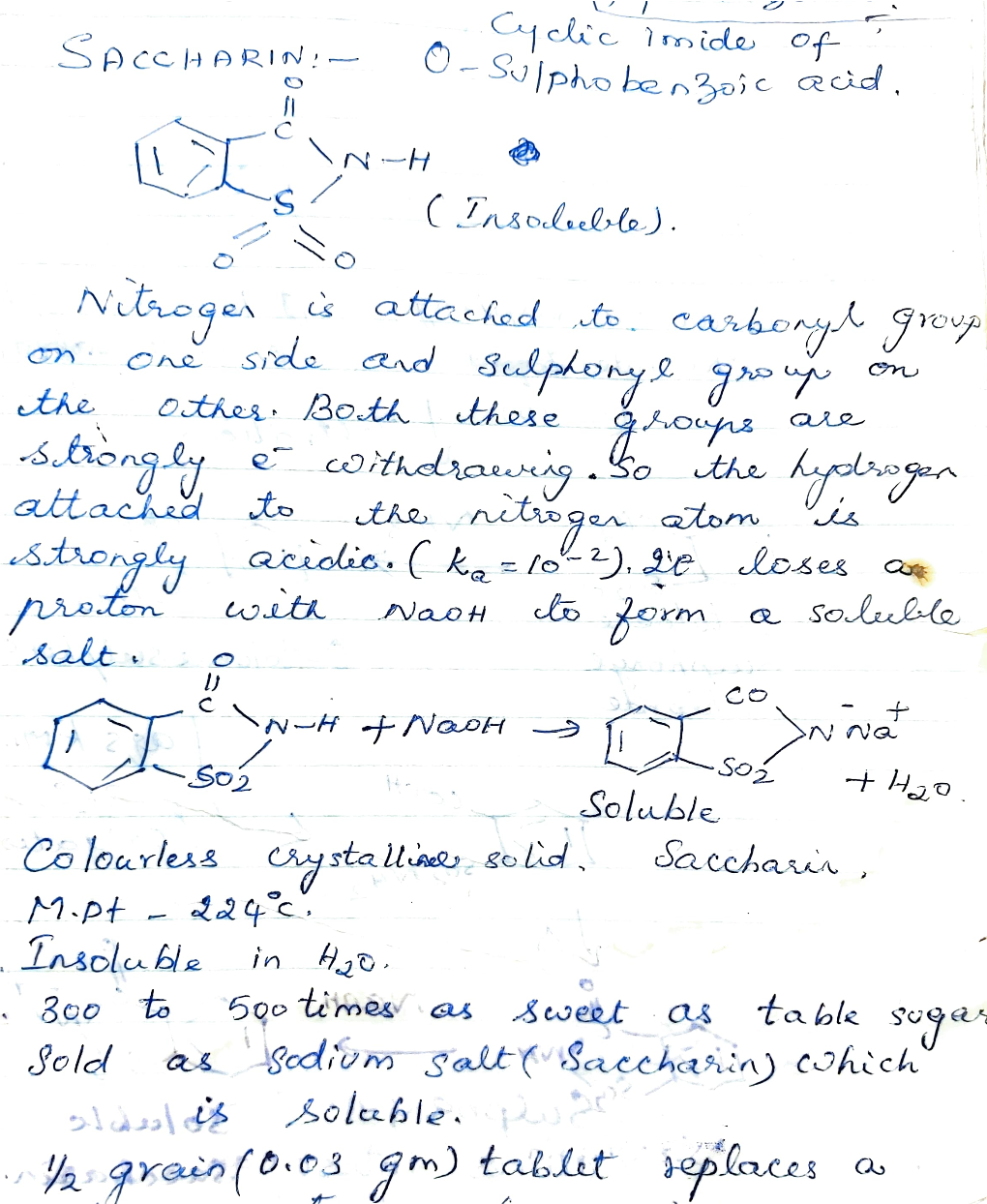
Js Soluble Vagrain (O.O3 Gm) Tablut Eplaces A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

All Proposals and Discussion Documents) (Pdf
National Organic Standards Board | October 2018| Proposals & Discussion Documents National Organic Standards Board Meeting Intercontinental Saint Paul Waterfront Hotel, Saint Paul, MN October 24-26, 2018 Title Page Materials Subcommittee (MS) | Harriet Behar, Chairperson Proposal: 2018 Research priorities 1 Proposal: Genetic integrity transparency of seed grown on organic land 11 Proposal: Excluded methods determinations October 2018 19 Discussion document: Marine materials in organic crop production 27 Compliance, Accreditation, & Certification Subcommittee (CACS) | Scott Rice, Chairperson Proposal: Developing criteria for risk-based accreditation oversight 39 Proposal: Training and oversight of inspector and certification reviewer personnel 43 Verbal update: Analysis of feedback from the Spring 2018 import panel No doc Livestock Subcommittee (LS) | Ashley Swaffar, Chairperson 2020 Sunset Reviews: Alcohols: ethanol, isopropanol; Aspirin; Biologics, vaccines; 47 Electrolytes; Glycerin; Phosphoric acid; Lime, hydrated; Mineral oil; Sucrose octanoate esters Discussion document: Oxalic acid - petitioned 63 Handling Subcommittee (HS) | Lisa de Lima, Chairperson 2020 Sunset Reviews: Calcium carbonate; Flavors; Gellan gum; Oxygen; Potassium 65 chloride; Alginates; Calcium hydroxide; Ethylene; Glycerides (mono and di); Magnesium stearate; Phosphoric acid; Potassium carbonate; Sulfur dioxide; Xanthan gum; Fructooligosaccharides (FOS); Gums: Arabic, Carob bean, Guar, Locust bean; Lecithin - de-oiled; Tragacanth gum Proposal: Sodium chlorite for -

ALCOHOLS, ETHERS and PHENOLS ALCOHOLS Molecules Containing –OH Group Are Termed As Alcohols
ALCOHOLS, ETHERS AND PHENOLS ALCOHOLS Molecules containing –OH group are termed as alcohols. Classification of alcohols they are classified as primary, secondary or tertiary alcohol according to the carbon that is bonded with –OH. Again when any molecule contains 1, 2 or 3 –OH groups then it is called mono, di or tri hydric alcohols respectively. (as in case of alkyl halides) OH OH OH OH OH CH32 – CH OH CH22 – CH CH22 – CH – CH Ethyl alcohol Ethylene glycol Glycerol (monohydric) (dihydric) (trihydric) Example 1: Classify the following into primary, secondary and tertiary alcohols: (a) CH3 (b) OH (c) HO OH H3C Solution: (a) Tertiary (b) Secondary (c) Tertiary GENERAL METHODS OF PREPARATION 1. From Alkenes : (i) By direct hydrolysis : OH H24 SO CH32 – CH = CH2 + H O CH33 – CH – CH (ii) Oxymercuration demercuration : OH OH NaBH4 Hg (OAc)2 CH – CH = CH + H O CH2 – CH – CH2 – CH33 – CH – CH 322 THF OH Hg(OAc) (iii) Hydroboration oxidation : OH BH26 H22 O /OH 6CH3 – CH = CH2 2(CH32 – CH – CH23 –) B 6CH3 – CH2 – CH 2 + 2H 3 BO 3 Overall result of above reaction is anti Markwonikoff addition of water and with no rearrangement. (iv) Oxo process followed by hydrogenation : O [Co(CO) ] 42 CH – CH – CH – C – H CH3 – CH = CH22 + CO + H high temperature 3 22 and high pressure H2 /Pd CH3 – CH 2 – CH 2 – CH 2 – OH Product has one more carbon. 2. From Alkyl Halides : When alkyl halides are treated with aq. KOH or aq. NaOH or moist Ag2O, alcohols are formed. – R X OHR OH X 3. -

Phenols & Phenolic Compounds
PHENOLS & PHENOLIC P COMPOUNDS A R I V E S CENTRAL POLLUTION CONTROL BOARD (Ministry of Environment, Forests & Climate Change) PariveshBhawan, East Arjun Nagar H Delhi - 110032 website: www.cpcb.nic.in AUGUST, 2016 1 FOREWORD Phenols or Phenolics are a family of organic compounds characterized by hydroxyl (-OH) group attached to an aromatic ring. Besides serving as generic name for the entire family, the term phenol (C6H5OH) itself is the first member commonly known as benzenol or carbolic acid. All other members in the family are known as derivatives of phenol and phenolic compounds. Phenolic compounds are common by-product of any industrial process viz. manufacture of dyes, plastics, drugs, antioxidants, paper and petroleum industries. Phenols and Phenolic compounds are widely used in household products and various industries, as intermediates during various industrial synthesis. Phenol itself is an established disinfectant in household cleaners. Phenols are used as basic material during production of plastics, explosives, drugs, Dye & Dye Intermediate Industries, commercial production of azo dyes etc. Phenolic resins form a large part of phenol production. Phenol formaldehyde resin was one of the earliest plastic known as Bakelite, which is still in use. Many phenolic compounds occur in nature and used in manufacture of perfumes and artificial flowers because of their pleasant odour and also have wide application in food as antioxidants. Because of wide use of phenols & phenolic compounds, these are discharged alongwith the effluents from several categories of industries such as Textiles, Woolen Mills, Dye & Dye Intermediate Industries, Coke ovens, Pulp & Paper Industries, Iron & Steel Plants, Petrochemicals, Paint Industries, Oil; Drilling & Gas Extraction units; Pharmaceuticals, Coal Washeries, Refractory Industries etc. -

Cropland Containers Chemical Resistance Chart
Cropland Containers Chemical Resistance Chart Fluorocarbon (Viton-type) A No known effect PTFE ( Teflon-type) B Some effect, evaluate with caution 316 Stainless Steel Polypropylene Nitrile / Buna N C Moderate to Severe effect, evaluation not recommended Polyethylene Carbon steel Stantoprene D Unknown EPDM Prodcut Description 1,2,2,2-Tetrafluoroethane, pure - Hydrocarbon, Aliphatic, Halogenated D D D D D D D 1,2-ethanediol, 30% - Alcohol, Aliphatic, Polyol A D D D D D D 1,2-ethanediol, pure - Alcohol, Aliphatic, Polyol A D D D D D D 1,2-propanediol, pure - Alcohol, Aliphatic, Polyol A D D D D D D 1,3-Benzenediol, 5% - Alcohol, Aromatic, Polyol A D D D D D D 1,3-Benzenediol, pure - Alcohol, Aromatic, Polyol A D D D D D D 1,3-Benzenediol, saturated - Alcohol, Aromatic, Polyol A D D D D D D 1,3-Butylene Glycol, pure - Alcohol, Aliphatic, Polyol D D D D D D D 1,4-benzoquinone, pure - Ketone, Aliphatic, Cyclic D D D D D D D 1-Dodecanol, pure - Alcohol, Aliphatic D D D D D D D 1-Hexadecanol, pure - Alcohol, Aliphatic A D D D D D D 1-Propanol, 100% - Alcohol, Aliphatic A D D D D D D 1-Propanol, pure - Alcohol, Aliphatic A D D D D D D 2,(2-Ethoxyethoxy)ethanol, pure - Ether, Aliphatic, Alcohol A D D D D D D 2,4,6-Trimethyl-1,3,5-trioxane, pure - Ketone, Aliphatic, Cyclic D D D D D D D 2-Butoxyethanol, pure - Ether, Aliphatic, Alcohol D D D D D D D 2-Mercaptoethanol, pure - Alcohol, Aliphatic, Mercaptan D D D D D D D 2-Methoxyethanol Acetate, pure - Ether, Aliphatic, Ester C D D D D D D 2-Methoxyethanol, pure - Ether, Aliphatic, Alcohol B D D D -

Doctoral Dissertation Template
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE THERMOGRAVIMETRY-MASS SPECTROMETRY AND VARIABLE TEMPERATURE INFRARED SPECTROSCOPY INVESTIGATIONS OF AROMATIC ACID INTERACTIONS WITH MONTMORILLONITE CLAYS AND ACID/BASE PROPERTIES OF DIIRON COORDINATION COMPLEXES A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY By AUDREY LYNN INGRAM Norman, Oklahoma 2015 THERMOGRAVIMETRY-MASS SPECTROMETRY AND VARIABLE TEMPERATURE INFRARED SPECTROSCOPY INVESTIGATIONS OF AROMATIC ACID INTERACTIONS WITH MONTMORILLONITE CLAYS AND ACID/BASE PROPERTIES OF DIIRON COORDINATION COMPLEXES A DISSERTATION APPROVED FOR THE DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY BY ______________________________ Dr. Robert L. White, Chair ______________________________ Dr. Mark A. Nanny ______________________________ Dr. Kenneth M. Nicholas ______________________________ Dr. Wai Tak Yip ______________________________ Dr. Zhibo Yang © Copyright by AUDREY LYNN INGRAM 2015 All Rights Reserved. For my wonderful husband, Dr. Kyle Ingram, and for my family: Dr. Dwight Myers, Nancy Myers, and Karen Myers. My achievements would not have been possible without them. Acknowledgements First and foremost, I would like to thank my mentor, Dr. Robert White, for all of his guidance and support. He is a brilliant chemist and teacher, and his enthusiasm for research and for helping his students to succeed are truly inspiring. I appreciate that he is always happy to take time to talk about the “why” instead of just caring about results. I would also like to thank my former research advisor, Dr. Robert Houser, for his guidance and patience as I learned how to perform research in the graduate setting. I appreciated that he always made time to talk about my project, even when he was busy with his departmental duties as Assistant Chair. -

National Organic Standards Board | April 2018| Proposals & Discussion Documents
National Organic Standards Board | April 2018| Proposals & Discussion Documents National Organic Standards Board Meeting Tucson University Hotel, Tucson, AZ April 25-27, 2018 Title Page Compliance, Accreditation, & Certification Subcommittee (CACS) | Scott Rice, Chairperson Discussion document: Import Oversight 1 Proposal: Inspector Qualifications 7 Proposal: Eliminating the incentive to convert native ecosystems to organic 49 production Livestock Subcommittee (LS) | Ashley Swaffar, Chairperson 2020 Sunset Reviews: Alcohols: ethanol, isopropanol; Aspirin; Biologics, vaccines; 53 Electrolytes; Glycerin; Phosphoric acid; Lime, hydrated; Mineral oil; Sucrose octanoate esters Proposal: Glycolic acid - petitioned 71 Proposal: Clarifying “emergency” for use of synthetic parasiticides in organic 81 livestock production Handling Subcommittee (HS) | Lisa de Lima, Chairperson 2020 Sunset Reviews: Calcium carbonate; Flavors; Gellan gum; Oxygen; 89 Potassium chloride; Alginates; Calcium hydroxide; Ethylene; Glycerides (mono and di); Magnesium stearate; Phosphoric acid; Potassium carbonate; Sulfur dioxide; Xanthan gum; Fructooligosaccharides (FOS); Gums: Arabic, Carob bean, Guar, Locust bean; Lecithin - de-oiled; Tragacanth gum Proposal: Sodium dodecylbenzene sulfonate (SDBS) - petitioned 123 Proposal: Magnesium chloride reclassification 205.605(b) 131 TOC 1 Crops Subcommittee (CS) | Steve Ela, Chairperson 2020 Sunset Reviews: Alcohols: ethanol, isopropanol; Sodium carbonate 133 peroxyhydrate ; Newspaper or other recycled paper ; Plastic mulch and