Dust Charging and Electrical Conductivity in the Day and Nighttime Atmosphere of Mars M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Mystery of Methane on Mars and Titan
The Mystery of Methane on Mars & Titan By Sushil K. Atreya MARS has long been thought of as a possible abode of life. The discovery of methane in its atmosphere has rekindled those visions. The visible face of Mars looks nearly static, apart from a few wispy clouds (white). But the methane hints at a beehive of biological or geochemical activity underground. Of all the planets in the solar system other than Earth, own way, revealing either that we are not alone in the universe Mars has arguably the greatest potential for life, either extinct or that both Mars and Titan harbor large underground bodies or extant. It resembles Earth in so many ways: its formation of water together with unexpected levels of geochemical activ- process, its early climate history, its reservoirs of water, its vol- ity. Understanding the origin and fate of methane on these bod- canoes and other geologic processes. Microorganisms would fit ies will provide crucial clues to the processes that shape the right in. Another planetary body, Saturn’s largest moon Titan, formation, evolution and habitability of terrestrial worlds in also routinely comes up in discussions of extraterrestrial biology. this solar system and possibly in others. In its primordial past, Titan possessed conditions conducive to Methane (CH4) is abundant on the giant planets—Jupiter, the formation of molecular precursors of life, and some scientists Saturn, Uranus and Neptune—where it was the product of chem- believe it may have been alive then and might even be alive now. ical processing of primordial solar nebula material. On Earth, To add intrigue to these possibilities, astronomers studying though, methane is special. -

Atmospheric Dust on Mars: a Review
47th International Conference on Environmental Systems ICES-2017-175 16-20 July 2017, Charleston, South Carolina Atmospheric Dust on Mars: A Review François Forget1 Laboratoire de Météorologie Dynamique (LMD/IPSL), Sorbonne Universités, UPMC Univ Paris 06, PSL Research University, Ecole Normale Supérieure, Université Paris-Saclay, Ecole Polytechnique, CNRS, Paris, France Luca Montabone2 Laboratoire de Météorologie Dynamique (LMD/IPSL), Paris, France & Space Science Institute, Boulder, CO, USA The Martian environment is characterized by airborne mineral dust extending between the surface and up to 80 km altitude. This dust plays a key role in the climate system and in the atmospheric variability. It is a significant issue for any system on the surface. The atmospheric dust content is highly variable in space and time. In the past 20 years, many investigations have been conducted to better understand the characteristics of the dust particles, their distribution and their variability. However, many unknowns remain. The occurrence of local, regional and global-scale dust storms are better documented and modeled, but they remain very difficult to predict. The vertical distribution of dust, characterized by detached layers exhibiting large diurnal and seasonal variations, remains quite enigmatic and very poorly modeled. Nomenclature Ls = Solar Longitude (°) MY = Martian year reff = Effective radius τ = Dust optical depth (or dust opacity) CDOD = Column Dust Optical Depth (i.e. τ integrated over the atmospheric column) IR = Infrared LDL = Low Dust Loading (season) HDL = High Dust Loading (season) MGS = Mars Global Surveyor (NASA spacecraft) MRO = Mars Reconnaissance Orbiter (NASA spacecraft) MEX = Mars Express (ESA spacecraft) TES = Thermal Emission Spectrometer (instrument aboard MGS) THEMIS = Thermal Emission Imaging System (instrument aboard NASA Mars Odyssey spacecraft) MCS = Mars Climate Sounder (instrument aboard MRO) PDS = Planetary Data System (NASA data archive) EDL = Entry, Descending, and Landing GCM = Global Climate Model I. -

Planetary Science
Mission Directorate: Science Theme: Planetary Science Theme Overview Planetary Science is a grand human enterprise that seeks to discover the nature and origin of the celestial bodies among which we live, and to explore whether life exists beyond Earth. The scientific imperative for Planetary Science, the quest to understand our origins, is universal. How did we get here? Are we alone? What does the future hold? These overarching questions lead to more focused, fundamental science questions about our solar system: How did the Sun's family of planets, satellites, and minor bodies originate and evolve? What are the characteristics of the solar system that lead to habitable environments? How and where could life begin and evolve in the solar system? What are the characteristics of small bodies and planetary environments and what potential hazards or resources do they hold? To address these science questions, NASA relies on various flight missions, research and analysis (R&A) and technology development. There are seven programs within the Planetary Science Theme: R&A, Lunar Quest, Discovery, New Frontiers, Mars Exploration, Outer Planets, and Technology. R&A supports two operating missions with international partners (Rosetta and Hayabusa), as well as sample curation, data archiving, dissemination and analysis, and Near Earth Object Observations. The Lunar Quest Program consists of small robotic spacecraft missions, Missions of Opportunity, Lunar Science Institute, and R&A. Discovery has two spacecraft in prime mission operations (MESSENGER and Dawn), an instrument operating on an ESA Mars Express mission (ASPERA-3), a mission in its development phase (GRAIL), three Missions of Opportunities (M3, Strofio, and LaRa), and three investigations using re-purposed spacecraft: EPOCh and DIXI hosted on the Deep Impact spacecraft and NExT hosted on the Stardust spacecraft. -

The Nakhlite Meteorites: Augite-Rich Igneous Rocks from Mars ARTICLE
ARTICLE IN PRESS Chemie der Erde 65 (2005) 203–270 www.elsevier.de/chemer INVITED REVIEW The nakhlite meteorites: Augite-rich igneous rocks from Mars Allan H. Treiman Lunar and Planetary Institute, 3600 Bay Area Boulevard, Houston, TX 77058-1113, USA Received 22 October 2004; accepted 18 January 2005 Abstract The seven nakhlite meteorites are augite-rich igneous rocks that formed in flows or shallow intrusions of basaltic magma on Mars. They consist of euhedral to subhedral crystals of augite and olivine (to 1 cm long) in fine-grained mesostases. The augite crystals have homogeneous cores of Mg0 ¼ 63% and rims that are normally zoned to iron enrichment. The core–rim zoning is cut by iron-enriched zones along fractures and is replaced locally by ferroan low-Ca pyroxene. The core compositions of the olivines vary inversely with the steepness of their rim zoning – sharp rim zoning goes with the most magnesian cores (Mg0 ¼ 42%), homogeneous olivines are the most ferroan. The olivine and augite crystals contain multiphase inclusions representing trapped magma. Among the olivine and augite crystals is mesostasis, composed principally of plagioclase and/or glass, with euhedra of titanomagnetite and many minor minerals. Olivine and mesostasis glass are partially replaced by veinlets and patches of iddingsite, a mixture of smectite clays, iron oxy-hydroxides and carbonate minerals. In the mesostasis are rare patches of a salt alteration assemblage: halite, siderite, and anhydrite/ gypsum. The nakhlites are little shocked, but have been affected chemically and biologically by their residence on Earth. Differences among the chemical compositions of the nakhlites can be ascribed mostly to different proportions of augite, olivine, and mesostasis. -

Reviewing Martian Atmospheric Noble Gas Measurements: from Martian Meteorites to Mars Missions
geosciences Review Reviewing Martian Atmospheric Noble Gas Measurements: From Martian Meteorites to Mars Missions Thomas Smith 1,* , P. M. Ranjith 1, Huaiyu He 1,2,3,* and Rixiang Zhu 1,2,3 1 State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, 19 Beitucheng Western Road, Box 9825, Beijing 100029, China; [email protected] (P.M.R.); [email protected] (R.Z.) 2 Institutions of Earth Science, Chinese Academy of Sciences, Beijing 100029, China 3 College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing 100049, China * Correspondence: [email protected] (T.S.); [email protected] (H.H.) Received: 10 September 2020; Accepted: 4 November 2020; Published: 6 November 2020 Abstract: Martian meteorites are the only samples from Mars available for extensive studies in laboratories on Earth. Among the various unresolved science questions, the question of the Martian atmospheric composition, distribution, and evolution over geological time still is of high concern for the scientific community. Recent successful space missions to Mars have particularly strengthened our understanding of the loss of the primary Martian atmosphere. Noble gases are commonly used in geochemistry and cosmochemistry as tools to better unravel the properties or exchange mechanisms associated with different isotopic reservoirs in the Earth or in different planetary bodies. The relatively low abundance and chemical inertness of noble gases enable their distributions and, consequently, transfer mechanisms to be determined. In this review, we first summarize the various in situ and laboratory techniques on Mars and in Martian meteorites, respectively, for measuring noble gas abundances and isotopic ratios. -

The Case for Rainfall on a Warm, Wet Early Mars Robert A
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. E11, 5111, doi:10.1029/2001JE001505, 2002 The case for rainfall on a warm, wet early Mars Robert A. Craddock Center for Earth and Planetary Studies, National Air and Space Museum, Smithsonian Institution, Washington, District of Columbia, USA Alan D. Howard Department of Environmental Sciences, University of Virginia, Charlottesville, Virginia, USA Received 11 April 2001; revised 10 April 2002; accepted 10 June 2002; published 23 November 2002. [1] Valley networks provide compelling evidence that past geologic processes on Mars were different than those seen today. The generally accepted paradigm is that these features formed from groundwater circulation, which may have been driven by differential heating induced by magmatic intrusions, impact melt, or a higher primordial heat flux. Although such mechanisms may not require climatic conditions any different than today’s, they fail to explain the large amount of recharge necessary for maintaining valley network systems, the spatial patterns of erosion, or how water became initially situated in the Martian regolith. In addition, there are no clear surface manifestations of any geothermal systems (e.g., mineral deposits or phreatic explosion craters). Finally, these models do not explain the style and amount of crater degradation. To the contrary, analyses of degraded crater morphometry indicate modification occurred from creep induced by rain splash combined with surface runoff and erosion; the former process appears to have continued late into Martian history. A critical analysis of the morphology and drainage density of valley networks based on Mars Global Surveyor data shows that these features are, in fact, entirely consistent with rainfall and surface runoff. -

Moon-Miners-Manifesto-Mars.Pdf
http://www.moonsociety.org/mars/ Let’s make the right choice - Mars and the Moon! Advantages of a low profile for shielding Mars looks like Arizona but feels like Antarctica Rover Opportunity at edge of Endeavor Crater Designing railroads and trains for Mars Designing planes that can fly in Mars’ thin air Breeding plants to be “Mars-hardy” Outposts between dunes, pulling sand over them These are just a few of the Mars-related topics covered in the past 25+ years. Read on for much more! Why Mars? The lunar and Martian frontiers will thrive much better as trading partners than either could on it own. Mars has little to trade to Earth, but a lot it can trade with the Moon. Both can/will thrive together! CHRONOLOGICAL INDEX MMM THEMES: MARS MMM #6 - "M" is for Missing Volatiles: Methane and 'Mmonia; Mars, PHOBOS, Deimos; Mars as I see it; MMM #16 Frontiers Have Rough Edges MMM #18 Importance of the M.U.S.-c.l.e.Plan for the Opening of Mars; Pavonis Mons MMM #19 Seizing the Reins of the Mars Bandwagon; Mars: Option to Stay; Mars Calendar MMM #30 NIMF: Nuclear rocket using Indigenous Martian Fuel; Wanted: Split personality types for Mars Expedition; Mars Calendar Postscript; Are there Meteor Showers on Mars? MMM #41 Imagineering Mars Rovers; Rethink Mars Sample Return; Lunar Development & Mars; Temptations to Eco-carelessness; The Romantic Touch of Old Barsoom MMM #42 Igloos: Atmosphere-derived shielding for lo-rem Martian Shelters MMM #54 Mars of Lore vs. Mars of Yore; vendors wanted for wheeled and walking Mars Rovers; Transforming Mars; Xities -

Unlocking the Climate Record Stored Within Mars' Polar Layered Deposits
Unlocking the Climate Record Stored within Mars' Polar Layered Deposits Final Report Keck Institute for Space Studies Isaac B. Smith York University and Planetary Science Instute Paul O. Hayne University of Colorado - Boulder Shane Byrne University of Arizona Pat Becerra University Bern Melinda Kahre NASA Ames Wendy Calvin University of Nevada - Reno Chrisne Hvidberg University of Copenhagen Sarah Milkovich Jet Propulsion Laboratory Peter Buhler Jet Propulsion Laboratory Margaret Landis Planetary Science Instute Briony Horgan Purdue University Armin Kleinböhl Jet Propulsion Laboratory Mahew Perry Planetary Science Instute Rachel Obbard Dartmouth University Jennifer Stern Goddard Space Flight Center Sylvain Piqueux Jet Propulsion Laboratory Nick Thomas University Bern Kris Zacny Honeybee Robocs Lynn Carter University of Arizona Lauren Edgar United States Geological Survey Jeremy EmmeM New Mexico State University Thomas Navarro University of California, Los Angeles Jennifer Hanley Lowell Observatory Michelle Koutnik University of Washington Nathaniel Putzig Planetary Science Instute Bryana L. Henderson Jet Propulsion Laboratory John W. Holt University of Arizona Bethany Ehlmann California Instute of Technology Sergio Parra Georgia Instute of Technology Daniel Lalich Cornell University Candy Hansen Planetary Science Instute Michael Hecht Haystack Observatory Don Banfield Cornell University Ken Herkenhoff United States Geological Survey David A. Paige University of California, Los Angeles Mark Skidmore Montana State University Robert L. Staehle Jet Propulsion Laboratory Mahew Siegler Planetary Science Instute 0. Execu)ve Summary 1. Background 1.A Mars Polar Science Overview and State of the Art 1.A.1 Polar ice deposits overview and present state 1.A.2 Polar Layered Deposits formaon and layers 1.A.3 Climate models and PLD formaon 1.A.4 Terrestrial Climate Studies Using Ice 1.B History of Mars Polar Invesgaons 1.B.1 Orbiters 1.B.2 Landers 1.B.3 Previous Concept Studies 2. -

The Present and Past Climates of Planet Mars
Eur. Phys. J. Conferences 1, 235–248 (2009) c EDP Sciences, 2009 THE EUROPEAN DOI: 10.1140/epjconf/e2009-0924-9 PHYSICAL JOURNAL CONFERENCES The present and past climates of planet Mars F. Forget LaboratoiredeM´et´eorologie Dynamique, IPSL, CNRS, Universit´e Paris 6, BP. 99, 75005 Paris, France Abstract. Mars is a small planet with a thin atmosphere of almost pure carbon dioxide. To first order, the Martian meteorology can be compared with what one would expect on a cold, dry desert-like Earth. However, several phenomena make the Martian climate system more complex than it appears. First, as much as 30% of the carbon dioxide atmosphere condenses every winter at high latitude to form CO2 ice polar caps, inducing large surface pressure variations all over the planet and an atmospheric circulation without equivalent on Earth. Second, a highly variable amount of suspended dust lifted by the winds modifies the radiative properties of the atmosphere, with sometime global dust storms able to totally shroud the planet. Last, a peculiar water cycle occurs on Mars, with water vapor transported by the atmosphere between the polar caps and possibly subsurface reservoirs, allowing the formation of clouds, hazes and frost. Telescopic and spacecraft observations have shown us that this complex climate system is highly variable, seasonally and from year to year, but these variations remain poorly understood. In fact, the Martian climate system has probably experienced large variations related to the oscillations in the parameters of the orbit and rotation of Mars (obliquity) a few millions or even thousand of years ago. -
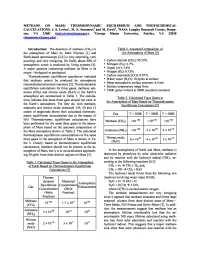
Methane on Mars: Thermodynamic Equilibrium and Photochemical Calculations
METHANE ON MARS: THERMODYNAMIC EQUILIBRIUM AND PHOTOCHEMICAL CALCULATIONS. J. S. Levine', M. E. Summers2 and M. Ewell2, 'NASA Langley Research Center, Hamp- ton, VA 2368' ([email protected] ), 2Geroge Mason University, Fairfax, VA 22030 ([email protected]) . Introduction: The detection of methane (CH4) in Table 2. Assumed Composition of the atmosphere of Mars by Mars Express [1] and the Atmosphere of Mars [ 5] Earth-based spectroscopy [2,3] is very surprising, very puzzling, and very intriguing. On Earth, about 90% of • Carbon dioxide (CO 2): 95.32% atmospheric ozone is produced by living systems [4]. • Nitrogen (N 2): 2.7% A major question concerning methane on Mars is its • Argon (Ar): 1.6% origin —biological or geological. • Oxygen (O 2): 0.13% Thermodynamic equilibrium caculations indicated • Carbon monoxide (CO): 0.07% that methane cannot be produced by atmospheric • Water vapor (H 2O): 10 ppmv at surface • Mean atmospheric surface pressure: 6.4 mb chemical/photochemical reactions [5]. Thermodynamic • Surface temperature range from equilibrium calculations for three gases, methane, am- • 148K (polar winter) to 290K (southern summer) monia (NH3) and nitrous oxide (N 2 O) in the Earth’s atmosphere are summarized in Table 1. The calcula- Table 3. Calculated Trace Gases in tions indicate that these three gses should not exist in the Atmosphere of Mars Based on Thermodynamic the Earth’s atmosphere. Yet they do, with methane, Equilibrium Calculations [5] ammonia and nitrous oxide enhanced 139, 50 and 12 orders of magnitude above their calculated thermody- Gas T=100KT= 200K T = 300K namic equilibrium concentration due to the impact of life! Thermodynamic equilibrium calculations have -100 -100 -100 Methane (CH4) <10 <10 <10 been performed for the same three gases in the atmos- phere of Mars based on the assumed composition of -100 -89 -62 the Mars atmosphere shown in Table 2. -

Spatial, Seasonal, and Solar Cycle Variations of the Martian Total
Journal of Geophysical Research: Planets RESEARCH ARTICLE Spatial, Seasonal, and Solar Cycle Variations 10.1029/2018JE005626 of the Martian Total Electron Content (TEC): Key Points: Is the TEC a Good Tracer for • The spatial, seasonal, and solar cycle variation of 10 years of Mars’ TEC is Atmospheric Cycles? assessed • Mars Express routinely measures Beatriz Sánchez-Cano1 , Mark Lester1 , Olivier Witasse2 , Pierre-Louis Blelly3, Mikel Indurain3, the dynamic of the 4 5 6 thermosphere-ionosphere coupling Marco Cartacci , Francisco González-Galindo , Álvaro Vicente-Retortillo , 4 4 • The TEC can be used as a tracer for Andrea Cicchetti , and Raffaella Noschese atmospheric cycles on the upper atmosphere 1Radio and Space Plasma Physics Group, Department of Physics and Astronomy, University of Leicester, Leicester, UK, 2European Space Agency, ESTEC-Scientific Support Office, Noordwijk, Netherlands, 3Institut de Recherche en Astrophysique et Planétologie, Toulouse, France, 4Istituto Nazionale di Astrofisica, Istituto di Astrofisica e Planetologia Spaziali, Rome, Italy, 5Instituto de Astrofísica de Andalucía, CSIC, Granada, Spain, 6Department of Climate and Space Correspondence to: B. Sánchez-Cano, Sciences and Engineering, University of Michigan, Ann Arbor, MI, USA [email protected] Abstract We analyze 10 years of Mars Express total electron content (TEC) data from the Mars Advanced Citation: Radar for Subsurface and Ionospheric Sounding (MARSIS) instrument. We describe the spatial, seasonal, Sánchez-Cano, B., Lester, M., Witasse, O., Blelly, -

Mars: Life, Subglacial Oceans, Abiogenic Photosynthesis, Seasonal Increases and Replenishment of Atmospheric Oxygen
Open Astron. 2020; 29: 189–209 Review Article Rhawn G. Joseph*, Natalia S. Duxbury, Giora J. Kidron, Carl H. Gibson, and Rudolph Schild Mars: Life, Subglacial Oceans, Abiogenic Photosynthesis, Seasonal Increases and Replenishment of Atmospheric Oxygen https://doi.org/10.1515/astro-2020-0020 Received Sep 3, 2020; peer reviewed and revised; accepted Oct 12, 2020 Abstract: The discovery and subsequent investigations of atmospheric oxygen on Mars are reviewed. Free oxygen is a biomarker produced by photosynthesizing organisms. Oxygen is reactive and on Mars may be destroyed in 10 years and is continually replenished. Diurnal and spring/summer increases in oxygen have been documented, and these variations parallel biologically induced fluctuations on Earth. Data from the Viking biological experiments also support active biology, though these results have been disputed. Although there is no conclusive proof of current or past life on Mars, organic matter has been detected and specimens resembling green algae / cyanobacteria, lichens, stromatolites, and open apertures and fenestrae for the venting of oxygen produced via photosynthesis have been observed. These life-like specimens include thousands of lichen-mushroom-shaped structures with thin stems, attached to rocks, topped by bulbous caps, and oriented skyward similar to photosynthesizing organisms. If these specimens are living, fossilized or abiogenic is unknown. If biological, they may be producing and replenishing atmospheric oxygen. Abiogenic processes might also contribute to oxygenation via sublimation and seasonal melting of subglacial water-ice deposits coupled with UV splitting of water molecules; a process of abiogenic photosynthesis that could have significantly depleted oceans of water and subsurface ice over the last 4.5 billion years.