Hyperchloremia and Moderate Increase in Serum Chloride Are Associated with Acute Kidney Injury in Severe Sepsis and Septic Shock
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Versus High-Chloride-Containing Hypertonic Solution for the Treatment
Sadan et al. Journal of Intensive Care (2020) 8:32 https://doi.org/10.1186/s40560-020-00449-0 RESEARCH Open Access Low-chloride- versus high-chloride- containing hypertonic solution for the treatment of subarachnoid hemorrhage– related complications: The ACETatE (A low ChloriE hyperTonic solution for brain Edema) randomized trial Ofer Sadan1*, Kai Singbartl2, Jacqueline Kraft1, Joao McONeil Plancher1, Alexander C. M. Greven3, Prem Kandiah1, Cederic Pimentel1, C. L. Hall1, Alexander Papangelou4, William H. Asbury5, John J. Hanfelt6 and Owen Samuels1 Abstract Background: Recent reports have demonstrated that among patients with subarachnoid hemorrhage (SAH) treated with hypertonic NaCl, resultant hyperchloremia has been associated with the development of acute kidney injury (AKI). We report a trial comparing the effect of two hypertonic solutions with different chloride contents on the resultant serum chloride concentrations in SAH patients, with a primary outcome aimed at limiting chloride elevation. Methods: A low ChloridE hyperTonic solution for brain Edema (ACETatE) trial is a single-center, double-blinded, double-dummy, randomized pilot trial comparing bolus infusions of 23.4% NaCl and 16.4% NaCl/Na-acetate for the treatment of cerebral edema in patients with SAH. Randomization occurred when patients developed hyperchloremia (serum Cl− ≥ 109 mmol/L) and required hyperosmolar treatment. Results: We enrolled 59 patients, of which 32 developed hyperchloremia and required hyperosmolar treatment. 15 patients were randomized to the 23.4% NaCl group, and 17 patients were randomized to the 16.4% NaCl/Na- acetate group. Although serum chloride levels increased similarly in both groups, the NaCl/Acetate group showed a significantly lower Cl− load at the end of the study period (978mEq vs. -

Clinical Physiology Aspects of Chloremia in Fluid Therapy: a Systematic Review David Astapenko1,2* , Pavel Navratil2,3, Jiri Pouska4,5 and Vladimir Cerny1,2,6,7,8,9
Astapenko et al. Perioperative Medicine (2020) 9:40 https://doi.org/10.1186/s13741-020-00171-3 REVIEW Open Access Clinical physiology aspects of chloremia in fluid therapy: a systematic review David Astapenko1,2* , Pavel Navratil2,3, Jiri Pouska4,5 and Vladimir Cerny1,2,6,7,8,9 Abstract Background: This systematic review discusses a clinical physiology aspect of chloride in fluid therapy. Crystalloid solutions are one of the most widely used remedies. While generally used in medicine for almost 190 years, studies focused largely on their safety have only been published since the new millennium. The most widely used solution, normal saline, is most often referred to in this context. Its excessive administration results in hyperchloremic metabolic acidosis with other consequences, including higher mortality rates. Methods: Original papers and review articles eligible for developing the present paper were identified by searching online in the electronic MEDLINE database. The keywords searched for included hyperchloremia, hypochloremia, and compound words containing the word “chloride,” infusion therapy, metabolic acidosis, renal failure, and review. Results: A total of 21,758 papers published before 31 May 2020 were identified; of this number, 630 duplicates were removed from the list. Upon excluding articles based on their title or abstract, 1850 papers were screened, of which 63 full-text articles were assessed. Conclusions: According to the latest medical concepts, dyschloremia (both hyperchloremia and hypochloremia) represents a factor indisputably having a negative effect on selected variables of clinical outcome. As infusion therapy can significantly impact chloride homeostasis of the body, the choice of infusion solutions should always take into account the potentially adverse impact of chloride content on chloremia and organ function. -

THE EFFECT of POTASSIUM CHLORIDE on HYPONATREMIA1 by JOHN H
THE EFFECT OF POTASSIUM CHLORIDE ON HYPONATREMIA1 By JOHN H. LARAGH2 (From the Department of Medicine, College of Physicians and Surgeons, Columbia University; and the Presbyterian Hospital in the City of New York) (Submitted for publication August 31, 1953; accepted February 10, 1954) In cardiac edema as well as in various other tration might favorably influence disturbances in states characterized by excessive retention of fluid sodium metabolism manifested by pathologic dis- there is observed frequently an abnormally low con- tribution of sodium and potassium within the body. centration of sodium in the serum and extracellular Two recent studies have served to emphasize this fluid (1, 2). Rigorous sodium restriction, mer- important relationship between sodium and potas- curial diuretics, and cation exchange resins may sium. In vivo, with potassium depletion there is exaggerate or produce this tendency to hypo- a movement of sodium ions into cells and an associ- tonicity. Sodium administration often enhances ated extracellular alkalosis. This intracellular so- accumulation of fluid, without increasing its to- dium can then be mobilized by potassium adminis- nicity, and hypertonic sodium chloride, though tration (11). In vitro it has been shown that po- effective at times, may be neither beneficial nor tassium transport is dependent upon energy of safe (3). aerobic oxidation. If the cell is injured or meta- Because of the shortcomings of these various bolically inhibited potassium fails to accumulate therapies and because hyponatremia per se may and is replaced by an influx of sodium and water play a role in the production of adverse symptoms, (12). it seemed desirable to search for another diuretic Accordingly, potassium chloride (KCl) was agent which might promote the loss of excessive given to an edematous, hyponatremic cardiac body water without aggravating disturbances in patient with the hope of effecting water diuresis. -

Renal Tubular Acidosis in Children: State of the Art, Diagnosis and Treatment
www.medigraphic.org.mx Bol Med Hosp Infant Mex 2013;70(3):178-193 REVIEW ARTICLE Renal tubular acidosis in children: state of the art, diagnosis and treatment Ricardo Muñoz-Arizpe,1 Laura Escobar,2 Mara Medeiros3 ABSTRACT Overdiagnosis of renal tubular acidosis (RTA) has been recently detected in Mexican children, perhaps due to diagnostic errors as well as due to a lack of knowledge regarding the pathophysiology and molecular biochemistry involved in this illness. The objective of the present study is to facilitate the knowledge and diagnosis of RTA, a clinical condition infrequently seen worldwide. RTA is an alteration of the acid-base equilibrium due to a bicarbonate wasting in the proximal renal tubules [proximal RTA, (pRTA) or type 2 RTA] or due to a distal nephron hy- drogen ion excretion defect [distal RTA (dRTA) or type 1 RTA]. Hyperkalemic, or type 4 RTA, is due to alterations in aldosterone metabolism. RTA may be primary, secondary, acquired or hereditary and frequently presents secondary to an array of systemic diseases, usually accom- panied by multiple renal tubular defects. The main defect occurs in the transmembrane transporters such as carbonic anhydrase (CA I and + - - + - II), H -ATPase, HCO3 /Cl (AE1) exchanger and Na /HCO3 (NBCe1) cotransporter. Diagnosis should include the presence of hyperchloremic metabolic acidosis with normal serum anion gap (done in an arterial or arterialized blood sample), lack of appetite, polyuria, thirst, growth failure, and rickets; nephrocalcinosis and renal stones (in dRTA); abnormal urine anion gap and abnormal urine/serum pCO2 gradient. Diagnosis of a primary systemic disease must be made in cases of secondary RTA. -

DKA Protocol - Insulin Deficiency - Pregnancy
Diabetic Ketoacidosis in Pregnancy Diagnosis of DKA: Initial STAT labs include • CBC with diff • Serum electrolytes • BUN • Creatinine • Glucose • Arterial blood gases • Bicarbonate • Urinalysis • Lactate • Serum ketones • Calculation of the Anion Gap serum anion gap = serum sodium – (serum chloride + bicarbonate) • Electrocardiogram Treatment Protocol for Diabetic Ketoacidosis Reviewed 5/2/2017 1 Updated 05/02/17 DKA/HHS Pathway Phase 1 (Adult) DKA Diagnostic Criteria: Blood glucose >250 mg/dl *PREGNANCY Arterial pH <7.3 Utilize OB DKA order set Phase 1 Bicarbonate ≤18 mEq/l When glucose reaches 200mg/dL, Initiate OB Anion Gap Acidosis DKA Phase 2 Moderate ketonuria or ketonemia Glucose goals 100 -150mg/dL OB DKA Phase 2 1. Start IV fluids (1 L of 0.9% NaCl per hr initially) Look for the Cause 2. If serum K+ is <3.3 mEq/L hold insulin - Infection/Inflammation (PNA, UTI, Give 40 mEq/h until K ≥ 3.3 mEq/L pancreatitis, cholecystitis) 3. Initiate DKA Order Set Phase I ( *In PREGNANCY utilize OB DKA - Ischemia/Infarction (myocardial, cerebral, gut) order set ) - Intoxication (EtOH, drugs) 4. Start insulin 0.14 units/kg/hr IV infusion (calculate dose) - Iatrogenic (drugs, lack of insulin) RN will titrate per DKA protocol - Insulin deficiency - Pregnancy IVF Insulin Potassium Bicarbonate + Determine hydration status Initiate and If initial serum K is Assess need for bicarbonate continue insulin gtt <3.3 mEq/L, hold until serum insulin and give 40 + glucose reaches mEq K per h (2/3 250 mg/dl. KCL and 1/3 KP0 ) Hypovolemic Mild Cardiogenic 4 pH <6.9 pH >7.0 shock hypotension shock RN will titrate per until K ≥ 3.3 mEq/L protocol to achieve target. -

Acid-Base Physiology & Anesthesia
ACID-BASE PHYSIOLOGY & ANESTHESIA Lyon Lee DVM PhD DACVA Introductions • Abnormal acid-base changes are a result of a disease process. They are not the disease. • Abnormal acid base disorder predicts the outcome of the case but often is not a direct cause of the mortality, but rather is an epiphenomenon. • Disorders of acid base balance result from disorders of primary regulating organs (lungs or kidneys etc), exogenous drugs or fluids that change the ability to maintain normal acid base balance. • An acid is a hydrogen ion or proton donor, and a substance which causes a rise in H+ concentration on being added to water. • A base is a hydrogen ion or proton acceptor, and a substance which causes a rise in OH- concentration when added to water. • Strength of acids or bases refers to their ability to donate and accept H+ ions respectively. • When hydrochloric acid is dissolved in water all or almost all of the H in the acid is released as H+. • When lactic acid is dissolved in water a considerable quantity remains as lactic acid molecules. • Lactic acid is, therefore, said to be a weaker acid than hydrochloric acid, but the lactate ion possess a stronger conjugate base than hydrochlorate. • The stronger the acid, the weaker its conjugate base, that is, the less ability of the base to accept H+, therefore termed, ‘strong acid’ • Carbonic acid ionizes less than lactic acid and so is weaker than lactic acid, therefore termed, ‘weak acid’. • Thus lactic acid might be referred to as weak when considered in relation to hydrochloric acid but strong when compared to carbonic acid. -

Hyperchloremia – Why and How
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):347–353 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Brief review Hyperchloremia – Why and how Glenn T. Nagami Nephrology Section, Department of Medicine, VA Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, United States a r t i c l e i n f o a b s t r a c t Article history: Hyperchloremia is a common electrolyte disorder that is associated with a diverse group of Received 5 April 2016 clinical conditions. The kidney plays an important role in the regulation of chloride concen- Accepted 11 April 2016 tration through a variety of transporters that are present along the nephron. Nevertheless, Available online 3 June 2016 hyperchloremia can occur when water losses exceed sodium and chloride losses, when the capacity to handle excessive chloride is overwhelmed, or when the serum bicarbonate is low Keywords: with a concomitant rise in chloride as occurs with a normal anion gap metabolic acidosis Hyperchloremia or respiratory alkalosis. The varied nature of the underlying causes of the hyperchloremia Electrolyte disorder will, to a large extent, determine how to treat this electrolyte disturbance. Serum bicarbonate Published by Elsevier Espana,˜ S.L.U. on behalf of Sociedad Espanola˜ de Nefrologıa.´ This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/). -

Should the Actual Or the Corrected Serum Sodium Be Used to Calculate the Anion Gap in Diabetic Ketoacidosis?
1-MINUTE CONSULT ONEONE MINUTE CONSULT BRIEF ANSWERS TO SPECIFIC Q: Should the actual or the corrected serum CLINICAL sodium be used to calculate the anion gap QUESTIONS in diabetic ketoacidosis? LAURENCE H. BECK, MD ic effect on the normal anion gap in the case Chairman, Department of Internal Medicine, Cleveland Clinic Florida, Fort Lauderdale of a 10% gain or 10% loss of water. Since all of the components of the anion gap calculation ONE SHOULD USE the actual, measured are concentrated or diluted to the same A: sodium concentration to calculate extent, the anion gap is minimally altered— the anion gap,1 and use the corrected sodium too little to interfere with proper interpreta- concentration to estimate the severity of tion of the acid-base status. dehydration in severe hyperglycemia. Use corrected sodium to evaluate dehydration Use the anion gap to evaluate ketoacidosis On the other hand, correcting the serum sodi- The anion gap (the serum sodium concentra- um concentration in patients with severe tion minus the sum of the serum chloride and hyperglycemia is very useful in estimating the bicarbonate concentrations) is useful in esti- magnitude of water loss that has occurred dur- mating the concentration of other anions not ing the development of hyperglycemia. The usually measured—notably, ketones such as most commonly used formula for correction is beta-hydroxybutyrate and acetoacetate. to add 1.6 mmol/L to the measured serum Why use the measured sodium and not sodium concentration for every 100-mg/dL Using the the corrected sodium? The anion gap reflects increase in glucose, although other conversion corrected the balance between positively and negatively factors have been suggested.2 charged constituents of extracellular fluid. -

The Prognostic Effects of Hyponatremia and Hyperchloremia on Postoperative NSCLC Patients
Current Problems in Cancer 43 (2019) 402–410 Contents lists available at ScienceDirect Current Problems in Cancer journal homepage: www.elsevier.com/locate/cpcancer The prognostic effects of hyponatremia and hyperchloremia on postoperative NSCLC patients Wei Li a,b,1, Xiaowei Chen a,1, Liguang Wang a,c, Yu Wang a, ∗ Cuicui Huang a, Guanghui Wang a,d, Jiajun Du a,d, a Institute of Oncology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China b Department of Thoracic Surgery, Shandong Juxian People’s Hospital, Rizhao, PR China c Department of Oncology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China d Department of Thoracic Surgery, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China a b s t r a c t Electrolytic disorders are common in lung cancer patients. But the association between serum electrolytes levels and survival in patients undergoing lung cancer resections for non–small-cell lung cancer (NSCLC) has been poorly inves- tigated. A retrospective study was conducted on consecutive postoperative NSCLC patients. Pearson’s test was used to determine the association between serum sodium and chlorine levels and clinical characteristics, and cox regression and Kaplan-Meier model were applied to analyze risk factors on overall survival. We found that hyponatremia was an independent prognostic factor associated with poor prognosis in NSCLC patients undergoing complete resection (log-rank test, P = 0.004). In addition, we found that hyperchloremia predicted a poor clinical outcome in patients with non-anion-gap (log-rank test, P = 0.011), whereas it predicted a favorable clinical outcome in patients with high- anion-gap (log-rank test, P = 0.002). -

Baseline Chloride Levels Are Associated with the Incidence Of
www.nature.com/scientificreports OPEN Baseline Chloride Levels are Associated with the Incidence of Contrast-Associated Acute Kidney Received: 26 June 2017 Accepted: 30 November 2017 Injury Published: xx xx xxxx Hyung Jung Oh1,2, Sungwon Kim3, Jung Tak Park4, Sang-Joon Kim5, Seung Hyeok Han 4, Tae-Hyun Yoo4, Dong-Ryeol Ryu 1,6,7, Shin-Wook Kang4,8 & Yong Eun Chung 3,8 Although hypo- and hyperchloremia have been associated with worsening renal outcomes, there has been no study that correlates hypo- and hyperchloremia and the incidence of contrast-associated acute kidney injury (CA-AKI). A total of 13,088 patients with less than 2.0 mg/dL of serum creatinine (Cr) who underwent contrast-enhanced abdominal CT (CECT) were included. Patients were divided into 3 groups based on Cl (the hypo-, normo- and hyperchloremia groups). Patients were also classifed by baseline Cr (<1.2; the ‘Normal Cr group’ and 1.2–2.0 mg/dL; the ‘Slightly increased Cr group’). Multivariate logistic regression analysis was used to reveal the association between Cl and CA-AKI. Among patients, 2,525 (19.3%) and 241 (1.8%) patients were classifed in the hypo- and hyperchloremia group. The incidence of CA-AKI was signifcantly lower in the normochloremia group (4.0%) compared to the hypo- (5.4%) and hyperchloremia groups (9.5%). On multivariate logistic regression, hypochloremia was signifcantly associated with the incidence of CA-AKI compared with normochloremia (1.382, P = 0.002). Moreover, hypochloremia was still signifcantly associated with the incidence of CA-AKI in ‘Normal Cr group’ compared with normochloremia (1.314, P = 0.015), while hyperchloremia did not show signifcant association with CA-AKI incidence. -

Effect of Blood Storage on Complete Biochemistry
isord D ers od & lo T r B f a n o s l f a u n s r Journal of i o u n o J Verma et al., J Blood Disord Transfus 2015, 6:6 ISSN: 2155-9864 Blood Disorders & Transfusion DOI: 10.4172/2155-9864.1000329 Research Article Open Access Effect of Blood Storage on Complete Biochemistry Monica Verma1*, Kiran Dahiya1, Deepika Malik1, Sehgal PK1, Rama Devi2, Abhishek Soni2 and Veena Singh Ghalaut1 1Department of Biochemistry, Pt. B.D. Sharma, University of Health Sciences, Rohtak, Haryana, India 2University of Health Sciences, Rohtak, Haryana, India *Corresponding author: Monica Verma, MBBS, MD (Biochemistry), Senior Resident, Department of Biochemistry, Pt. B.D. Sharma, University of Health Sciences, 236- G, Model Town, Rohtak, Haryana-124001, India, Tel: +919813334543; E-mail: [email protected] Received date: Oct 12, 2015, Accepted date: Dec 28, 2015, Publication date: Dec 31, 2015 Copyright: © 2015 Verma M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Background: Prolonged storage of blood leads to alteration in RBCs biochemistry which may lose viability with time. Aim: This study was planned to observe biochemical changes on stored blood on 19 different analytes. Material and Methods: The study was conducted on blood donated by 30 healthy volunteer donors. Effect of storage was analyzed at 0, 3, 7, 14 and 21 days interval. Biochemical parameters were measured using Randox suzuka autoanalyzer and Combiline ISE analyzer. -
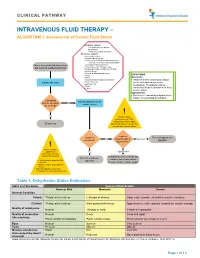
Intravenous Fluid Therapy – Algorithm 1
CLINICAL PATHWAY INTRAVENOUS FLUID THERAPY – ALGORITHM 1. Assessment of Overall Fluid Status Inclusion criteria: • All inpatients except those listed below • Patients pending admission Exclusion criteria: •Acute kidney injury •Chronic kidney disease •Endocrine or renal abnormalities leading to electrolyte derangements including DKA Patient who meets inclusion criteria •Oncology treatment protocol •Patients less than 30 days of age, and warrants supplemental fluids Including premature infants corrected for gestational age •Increased intracranial pressure •PICU DEFINITIONS •NICU Euvolemic: •Total Parenteral Nutrition dependent • Patient is at their ideal volume status Obtain vital signs •Pyloric Stenosis (neither dehydrated nor volume •Burn patients overloaded). The patient requires •Shock •Codes intravenous fluids to maintain their ideal volume status. Hypovolemic: • Patient is at least mildly dehydrated (see Table 1 for estimating dehydration) Can patient Assess patient’s current tolerate adequate No volume status enteral fluids? ! Yes Volume status assessment is 100% clinical. Do not rely upon Off pathway laboratory values to determine the patient’s volume status. Is patient Is patient Refer to hypovolemic No hypervolemic or Hypovolemic euvolemic? algorithm hypovolemic? ! Prior to starting a patient on Yes Hypervolemic maintenance IV fluids, consider the following: Reassess need for IV fluids and Refer to euvolemic consider issues with oncotic • Risk factors for abnormal ADH algorithm secretion pressure and/or cardiac output • Initial