Rapid Population Decline in Migratory Shorebirds Relying on Yellow Sea Tidal Mudflats As Stopover Sites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
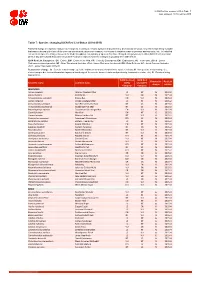
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Nordmann's Greenshank Population Analysis, at Pantai Cemara Jambi
Final Report Nordmann’s Greenshank Population Analysis, at Pantai Cemara Jambi Cipto Dwi Handono1, Ragil Siti Rihadini1, Iwan Febrianto1 and Ahmad Zulfikar Abdullah1 1Yayasan Ekologi Satwa Alam Liar Indonesia (Yayasan EKSAI/EKSAI Foundation) Surabaya, Indonesia Background Many shorebirds species have declined along East Asian-Australasian Flyway which support the highest diversity of shorebirds in the world, including the globally endangered species, Nordmann’s Greenshank. Nordmann’s Greenshank listed as endangered in the IUCN Red list of Threatened Species because of its small and declining population (BirdLife International, 2016). It’s one of the world’s most threatened shorebirds, is confined to the East Asian–Australasian Flyway (Bamford et al. 2008, BirdLife International 2001, 2012). Its global population is estimated at 500–1,000, with an estimated 100 in Malaysia, 100–200 in Thailand, 100 in Myanmar, plus unknown but low numbers in NE India, Bangladesh and Sumatra (Wetlands International 2006). The population is suspected to be rapidly decreasing due to coastal wetland development throughout Asia for industry, infrastructure and aquaculture, and the degradation of its breeding habitat in Russia by grazing Reindeer Rangifer tarandus (BirdLife International 2012). Mostly Nordmann’s Greenshanks have been recorded in very small numbers throughout Southeast Asia, and there are few places where it has been reported regularly. In Myanmar, for example, it was rediscovered after a gap of almost 129 years. The total count recorded by the Asian Waterbird Census (AWC) in 2006 for Myanmar was 28 birds with 14 being the largest number at a single locality (Naing 2007). In 2011–2012, Nordmann’s Greenshank was found three times in Sumatera Utara province, N Sumatra. -

Migratory Shorebirds Management Plan
Report GLNG Curtis Island Marine Facilities Migratory Shorebirds Environmental Management Plan 17 MARCH 2011 Prepared for GLNG Operations Pty Ltd Level 22 Santos Place 32 Turbot Street Brisbane Qld 4000 42626727 Project Manager: URS Australia Pty Ltd Level 16, 240 Queen Street Angus McLeod Brisbane, QLD 4000 Senior Ecologist GPO Box 302, QLD 4001 Australia T: 61 7 3243 2111 Principal-In-Charge: F: 61 7 3243 2199 Chris Pigott Senior Principal Author: Angus McLeod Senior Ecologist Reviewer: Date: 17 March 2011 Reference: 42626727/01/03 Status: Final Chris Pratt Principal Environmental Scientist j:\jobs\42626727\5 works\draft emp\for tina 17.3.11\3310-glng-3-3 3-0065_shorebirds_final_17 03 2011.doc Table of Contents Abbreviations............................................................................................................iii Executive Summary..................................................................................................iv 1 Introduction .......................................................................................................1 1.1 Project Background .........................................................................................1 1.2 Purpose of the Migratory Shorebirds Environment Management Plan ...................................................................................................................1 1.3 Aims and Objectives ........................................................................................3 1.4 Study Area ........................................................................................................3 -

Long-Billed Curlew Distributions in Intertidal Habitats: Scale-Dependent Patterns Ryan L
LONG-BILLED CURLEW DISTRIBUTIONS IN INTERTIDAL HABITATS: SCALE-DEPENDENT PATTERNS RYAN L. MATHIS, Department of Wildlife, Humboldt State University, Arcata, Cali- fornia 95521 (current address: National Wild Turkey Federation, P. O. Box 1050, Arcata, California 95518) MARK A. ColwELL, Department of Wildlife, Humboldt State University, Arcata, California 95521; [email protected] LINDA W. LEEMAN, Department of Wildlife, Humboldt State University, Arcata, California 95521 (current address: EDAW, Inc., 2022 J. St., Sacramento, California 95814) THOMAS S. LEEMAN, Department of Wildlife, Humboldt State University, Arcata, California 95521 (current address: Environmental Science Associates, 8950 Cal Center Drive, Suite 300, Sacramento, California 95826) ABSTRACT. Key ecological insights come from understanding a species’ distribu- tion, especially across several spatial scales. We studied the distribution (uniform, random, or aggregated) at low tide of nonbreeding Long-billed Curlew (Numenius americanus) at three spatial scales: within individual territories (1–8 ha), in the Elk River estuary (~50 ha), and across tidal habitats of Humboldt Bay (62 km2), Cali- fornia. During six baywide surveys, 200–300 Long-billed Curlews were aggregated consistently in certain areas and were absent from others, suggesting that foraging habitats varied in quality. In the Elk River estuary, distributions were often (73%) uniform as curlews foraged at low tide, although patterns tended toward random (27%) when more curlews were present during late summer and autumn. Patterns of predominantly uniform distribution across the estuary were a consequence of ter- ritoriality. Within territories, eight Long-billed Curlews most often (75%) foraged in a manner that produced a uniform distribution; patterns tended toward random (16%) and aggregated (8%) when individuals moved over larger areas. -

Population Analysis and Community Workshop for Far Eastern Curlew Conservation Action in Pantai Cemara, Desa Sungai Cemara – Jambi
POPULATION ANALYSIS AND COMMUNITY WORKSHOP FOR FAR EASTERN CURLEW CONSERVATION ACTION IN PANTAI CEMARA, DESA SUNGAI CEMARA – JAMBI Final Report Small Grant Fund of the EAAFP Far Eastern Curlew Task Force Iwan Febrianto, Cipto Dwi Handono & Ragil S. Rihadini Jambi, Indonesia 2019 The aim of this project are to Identify the condition of Far Eastern Curlew Population and the remaining potential sites for Far Eastern Curlew stopover in Sumatera, Indonesia and protect the remaining stopover sites for Far Eastern Curlew by educating the government, local people and community around the sites as the effort of reducing the threat of habitat degradation, habitat loss and human disturbance at stopover area. INTRODUCTION The Far Eastern Curlew (Numenius madagascariencis) is the largest shorebird in the world and is endemic to East Asian – Australian Flyway. It is one of the Endangered migratory shorebird with estimated global population at 38.000 individual, although a more recent update now estimates the population at 32.000 (Wetland International, 2015 in BirdLife International, 2017). An analysis of monitoring data collected from around Australia and New Zealand (Studds et al. in prep. In BirdLife International, 2017) suggests that the species has declined much more rapidly than was previously thought; with an annual rate of decline of 0.058 equating to a loss of 81.7% over three generations. Habitat loss occuring as a result of development is the most significant threat currently affecting migratory shorebird along the EAAF (Melville et al. 2016 in EAAFP 2017). Loss of habitat at critical stopover sites in the Yellow Sea is suspected to be the key threat to this species and given that it is restricted to East Asian - Australasian Flyway, the declines in the non-breeding are to be representative of the global population. -

<I>Actitis Hypoleucos</I>
Partial primary moult in first-spring/summer Common Sandpipers Actitis hypoleucos M. NICOLL 1 & P. KEMP 2 •c/o DundeeMuseum, Dundee, Tayside, UK 243 LochinverCrescent, Dundee, Tayside, UK Citation: Nicoll, M. & Kemp, P. 1983. Partial primary moult in first-spring/summer Common Sandpipers Actitis hypoleucos. Wader Study Group Bull. 37: 37-38. This note is intended to draw the attention of wader catch- and the old inner feathersare often retained (Pearson 1974). ers to the needfor carefulexamination of the primariesof Similarly, in Zimbabwe, first-year Common Sandpipers CommonSandpipers Actiris hypoleucos,and other waders, replacethe outerfive to sevenprimaries between December for partial primarywing moult. This is thoughtto be a diag- andApril (Tree 1974). It thusseems normal for first-spring/ nosticfeature of wadersin their first spring and summer summerCommon Sandpipers wintering in eastand southern (Tree 1974). Africa to show a contrast between new outer and old inner While membersof the Tay Ringing Group were mist- primaries.There is no informationfor birdswintering further nettingin Angus,Scotland, during early May 1980,a Com- north.However, there may be differencesin moult strategy mon Sandpiperdied accidentally.This bird was examined betweenwintering areas,since 3 of 23 juvenile Common and measured, noted as an adult, and then stored frozen un- Sandpiperscaught during autumn in Morocco had well- til it was skinned,'sexed', andthe gut contentsremoved for advancedprimary moult (Pienkowski et al. 1976). These analysis.Only duringskinning did we noticethat the outer birdswere moultingnormally, and so may have completed primarieswere fresh and unworn in comparisonto the faded a full primary moult during their first winter (M.W. Pien- and abradedinner primaries.The moult on both wingswas kowski, pers.comm.). -

Draft Version Target Shorebird Species List
Draft Version Target Shorebird Species List The target species list (species to be surveyed) should not change over the course of the study, therefore determining the target species list is an important project design task. Because waterbirds, including shorebirds, can occur in very high numbers in a census area, it is often not possible to count all species without compromising the quality of the survey data. For the basic shorebird census program (protocol 1), we recommend counting all shorebirds (sub-Order Charadrii), all raptors (hawks, falcons, owls, etc.), Common Ravens, and American Crows. This list of species is available on our field data forms, which can be downloaded from this site, and as a drop-down list on our online data entry form. If a very rare species occurs on a shorebird area survey, the species will need to be submitted with good documentation as a narrative note with the survey data. Project goals that could preclude counting all species include surveys designed to search for color-marked birds or post- breeding season counts of age-classed bird to obtain age ratios for a species. When conducting a census, you should identify as many of the shorebirds as possible to species; sometimes, however, this is not possible. For example, dowitchers often cannot be separated under censuses conditions, and at a distance or under poor lighting, it may not be possible to distinguish some species such as small Calidris sandpipers. We have provided codes for species combinations that commonly are reported on censuses. Combined codes are still species-specific and you should use the code that provides as much information as possible about the potential species combination you designate. -

Purple Sandpiper
Maine 2015 Wildlife Action Plan Revision Report Date: January 13, 2016 Calidris maritima (Purple Sandpiper) Priority 1 Species of Greatest Conservation Need (SGCN) Class: Aves (Birds) Order: Charadriiformes (Plovers, Sandpipers, And Allies) Family: Scolopacidae (Curlews, Dowitchers, Godwits, Knots, Phalaropes, Sandpipers, Snipe, Yellowlegs, And Woodcock) General comments: Recent surveys suggest population undergoing steep population decline within 10 years. IFW surveys conducted in 2014 suggest population declined by 49% since 2004 (IFW unpublished data). Maine has high responsibility for wintering population, regional surveys suggest Maine may support over 1/3 of the Western Atlantic wintering population. USFWS Region 5 and Canadian Maritimes winter at least 90% of the Western Atlantic population. Species Conservation Range Maps for Purple Sandpiper: Town Map: Calidris maritima_Towns.pdf Subwatershed Map: Calidris maritima_HUC12.pdf SGCN Priority Ranking - Designation Criteria: Risk of Extirpation: NA State Special Concern or NMFS Species of Concern: NA Recent Significant Declines: Purple Sandpiper is currently undergoing steep population declines, which has already led to, or if unchecked is likely to lead to, local extinction and/or range contraction. Notes: Recent surveys suggest population undergoing steep population decline within 10 years. IFW surveys conducted in 2014 suggest population declined by 49% since 2004 (IFW unpublished data). Maine has high responsibility for wintering populat Regional Endemic: Calidris maritima's global geographic range is at least 90% contained within the area defined by USFWS Region 5, the Canadian Maritime Provinces, and southeastern Quebec (south of the St. Lawrence River). Notes: Recent surveys suggest population undergoing steep population decline within 10 years. IFW surveys conducted in 2014 suggest population declined by 49% since 2004 (IFW unpublished data). -

Birds of the East Texas Baptist University Campus with Birds Observed Off-Campus During BIOL3400 Field Course
Birds of the East Texas Baptist University Campus with birds observed off-campus during BIOL3400 Field course Photo Credit: Talton Cooper Species Descriptions and Photos by students of BIOL3400 Edited by Troy A. Ladine Photo Credit: Kenneth Anding Links to Tables, Figures, and Species accounts for birds observed during May-term course or winter bird counts. Figure 1. Location of Environmental Studies Area Table. 1. Number of species and number of days observing birds during the field course from 2005 to 2016 and annual statistics. Table 2. Compilation of species observed during May 2005 - 2016 on campus and off-campus. Table 3. Number of days, by year, species have been observed on the campus of ETBU. Table 4. Number of days, by year, species have been observed during the off-campus trips. Table 5. Number of days, by year, species have been observed during a winter count of birds on the Environmental Studies Area of ETBU. Table 6. Species observed from 1 September to 1 October 2009 on the Environmental Studies Area of ETBU. Alphabetical Listing of Birds with authors of accounts and photographers . A Acadian Flycatcher B Anhinga B Belted Kingfisher Alder Flycatcher Bald Eagle Travis W. Sammons American Bittern Shane Kelehan Bewick's Wren Lynlea Hansen Rusty Collier Black Phoebe American Coot Leslie Fletcher Black-throated Blue Warbler Jordan Bartlett Jovana Nieto Jacob Stone American Crow Baltimore Oriole Black Vulture Zane Gruznina Pete Fitzsimmons Jeremy Alexander Darius Roberts George Plumlee Blair Brown Rachel Hastie Janae Wineland Brent Lewis American Goldfinch Barn Swallow Keely Schlabs Kathleen Santanello Katy Gifford Black-and-white Warbler Matthew Armendarez Jordan Brewer Sheridan A. -

Tringarefs V1.3.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2016 & 2017 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and best employed in conjunction with other reference sources. Grateful thanks to Graham Clarke (http://grahamsphoto.blogspot.com/) and Tom Shevlin (www.wildlifesnaps.com) for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds). 2016. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 6.1 accessed February 2016]). Version Version 1.3 (March 2016). Cover Main image: Spotted Redshank. Albufera, Mallorca. 13th April 2011. Picture by Graham Clarke. Vignette: Solitary Sandpiper. Central Bog, Cape Clear Island, Co. Cork, Ireland. 29th August 2008. Picture by Tom Shevlin. Species Page No. Greater Yellowlegs [Tringa melanoleuca] 14 Green Sandpiper [Tringa ochropus] 16 Greenshank [Tringa nebularia] 11 Grey-tailed Tattler [Tringa brevipes] 20 Lesser Yellowlegs [Tringa flavipes] 15 Marsh Sandpiper [Tringa stagnatilis] 10 Nordmann's Greenshank [Tringa guttifer] 13 Redshank [Tringa totanus] 7 Solitary Sandpiper [Tringa solitaria] 17 Spotted Redshank [Tringa erythropus] 5 Wandering Tattler [Tringa incana] 21 Willet [Tringa semipalmata] 22 Wood Sandpiper [Tringa glareola] 18 1 Relevant Publications Bahr, N. 2011. The Bird Species / Die Vogelarten: systematics of the bird species and subspecies of the world. Volume 1: Charadriiformes. Media Nutur, Minden. Balmer, D. et al 2013. Bird Atlas 2001-11: The breeding and wintering birds of Britain and Ireland. -

Breeding Birds of the Texas Coast
Roseate Spoonbill • L 32”• Uncom- Why Birds are Important of the mon, declining • Unmistakable pale Breeding Birds Texas Coast pink wading bird with a long bill end- • Bird abundance is an important indicator of the ing in flat “spoon”• Nests on islands health of coastal ecosystems in vegetation • Wades slowly through American White Pelican • L 62” Reddish Egret • L 30”• Threatened in water, sweeping touch-sensitive bill •Common, increasing • Large, white • Revenue generated by hunting, photography, and Texas, decreasing • Dark morph has slate- side to side in search of prey birdwatching helps support the coastal economy in bird with black flight feathers and gray body with reddish breast, neck, and Chuck Tague bright yellow bill and pouch • Nests Texas head; white morph completely white – both in groups on islands with sparse have pink bill with Black-bellied Whistling-Duck vegetation • Preys on small fish in black tip; shaggy- • L 21”• Lo- groups looking plumage cally common, increasing • Goose-like duck Threats to Island-Nesting Bay Birds Chuck Tague with long neck and pink legs, pinkish-red bill, Greg Lavaty • Nests in mixed- species colonies in low vegetation or on black belly, and white eye-ring • Nests in tree • Habitat loss from erosion and wetland degradation cavities • Occasionally nests in mesquite and Brown Pelican • L 51”• Endangered in ground • Uses quick, erratic movements to • Predators such as raccoons, feral hogs, and stir up prey Chuck Tague other woody vegetation on bay islands Texas, but common and increasing • Large -
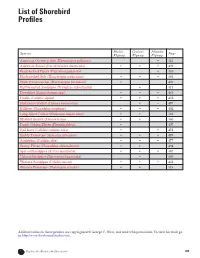
List of Shorebird Profiles
List of Shorebird Profiles Pacific Central Atlantic Species Page Flyway Flyway Flyway American Oystercatcher (Haematopus palliatus) •513 American Avocet (Recurvirostra americana) •••499 Black-bellied Plover (Pluvialis squatarola) •488 Black-necked Stilt (Himantopus mexicanus) •••501 Black Oystercatcher (Haematopus bachmani)•490 Buff-breasted Sandpiper (Tryngites subruficollis) •511 Dowitcher (Limnodromus spp.)•••485 Dunlin (Calidris alpina)•••483 Hudsonian Godwit (Limosa haemestica)••475 Killdeer (Charadrius vociferus)•••492 Long-billed Curlew (Numenius americanus) ••503 Marbled Godwit (Limosa fedoa)••505 Pacific Golden-Plover (Pluvialis fulva) •497 Red Knot (Calidris canutus rufa)••473 Ruddy Turnstone (Arenaria interpres)•••479 Sanderling (Calidris alba)•••477 Snowy Plover (Charadrius alexandrinus)••494 Spotted Sandpiper (Actitis macularia)•••507 Upland Sandpiper (Bartramia longicauda)•509 Western Sandpiper (Calidris mauri) •••481 Wilson’s Phalarope (Phalaropus tricolor) ••515 All illustrations in these profiles are copyrighted © George C. West, and used with permission. To view his work go to http://www.birchwoodstudio.com. S H O R E B I R D S M 472 I Explore the World with Shorebirds! S A T R ER G S RO CHOOLS P Red Knot (Calidris canutus) Description The Red Knot is a chunky, medium sized shorebird that measures about 10 inches from bill to tail. When in its breeding plumage, the edges of its head and the underside of its neck and belly are orangish. The bird’s upper body is streaked a dark brown. It has a brownish gray tail and yellow green legs and feet. In the winter, the Red Knot carries a plain, grayish plumage that has very few distinctive features. Call Its call is a low, two-note whistle that sometimes includes a churring “knot” sound that is what inspired its name.