Genetic Variability Studies in Response to Drought Under Different Water Regimes in Muskmelon (Cucumis Melo L.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Profile of Dr.Ranvinderjit Kaur
PROFILE OF DR.RANVINDERJIT KAUR PRESENT DESIGNATION: Assistant Professor, Department of Public Administration, Punjabi University, Patiala (Pb) EMAIL: [email protected] TEACHNG EXPERIENCE AT UNIVERSITY LEVEL: 7 YEARS DATE OF BIRTH: 18th JULY, 1985 ACADEMIC QUALIFICATIONS: DEGREE SUBJECTS/TITLE UNIVERSITY YEAR B.A Psychology, Economics, PUNJABI UNIVERSITY 2005 Political Science M.A(Distinction) Public Administration PUNJABI UNIVERSITY 2007 Ph.D(UGC JRF Problem of Child Labour PUNJABI UNIVERSITY 2012 QUALIFIED) in Punjab: A Case Study of Barnala and Sangrur Districts. (Pubic Administration) SPECIALIZATION: 1. Social Welfare Administration (Major) 2. Local Government (Minor) RESEARCH GUIDANCE: Ph.D - 12 (pursuing) M.phil -3 (Degree awarded) 7(pursuing) Diploma- 12 (Degree awarded) WORKSHOPS/SEMINARS/CONFERENCES(NATIONAL/ITERNATIONAL) ORGANISED SEMINARS/CONFERENCES (INTERNATIONAL/NATIONAL): 1. Ranvinderjit Kaur, “Role of Intergovernmental Agencies in Indian Federalism”, on ‘Changing Nature of Indian Federalism since the Last Decade’, Organized by Department of Political Science, Hindu Girls College, Jagadhri (29-30 September 2008). 2. Ranvinderjit Kaur, “Administrative Machinery for the Protection of Consumer with Special Focus on Punjab”, on ‘Consumer Protection in India’, Held at Maharishi Dayanand University, Rohtak (5-6 November 2009). 3. Ranvinderjit Kaur, “Good Governance and Panchayati Raj Institutions”, on ‘Good Governance’, Held at Public Administration University of Lucknow (5-6 December 2009). 4. Ranvinderjit Kaur, “Panchayati Raj Institution in India: Need for Reforms”, on ‘Public Administration in 21st Century: Major Trends & Challenges”, Organized by Department of Public Administration, Punjabi University Patiala (16-17 February 2009). 5. Ranvinderjit Kaur, “Role of ICT in Distance Education”, Organized by Department of Computer Science & Application, Hindu Girls College, Jagadhri (14-15 September 2010). -
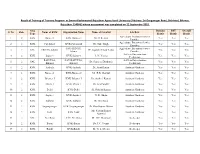
Result of Training of Trainers Program at Swami Keshwanand Rajasthan
Result of Training of Trainers Program at Swami Keshwanand Rajasthan Agricultural University [Address: Sri Ganganagar Road, Bichhwal, Bikaner, Rajasthan 334006] whose assessment was completed on 22 September 2018. Org Domain PST Overall S. No Zone Name of KVK Organization Name Name of Scientist Job Role Type Result Result Result Agriculture Extension Service 1 2 KVK Barmer-I KVK-Barmer-I Mr. H.R. Sain Yes Yes Yes Provider Agriculture Extension Service 2 2 KVK Fatehabad KVK-Fatehabad Dr. Sube Singh Yes Yes Yes Provider SAU-SKNAU, Agriculture Extension Service 3 2 SAU SKNAU, Jobner Dr. Jagdish Prasad Yadav Yes Yes Yes Jobner Provider Artificial Insemination 4 2 KVK Jaipur-1 KVK-Jaipur-1 L. N. Verma Yes Yes Yes Technician RAJUVAS, SAU-RAJUVAS, Artificial Insemination 5 2 SAU Dr. Sandeep Dholpuria Yes Yes Yes Bikaner Bikaner Technician 6 2 KVK Ambala KVK-Ambala Dr. Amit Kumar Assistant Gardener Yes Yes Yes 7 2 KVK Barmer-I KVK-Barmer-I Mr. B.R. Morwal Assistant Gardener Yes Yes Yes 8 2 KVK Bikaner-I KVK-Bikaner-I Dr. Susheel Kumar Assistant Gardener Yes Yes Yes 9 2 KVK Churu-I KVK-Churu-I Mr. G S Pundhir Assistant Gardener Yes Yes Yes 10 2 KVK Delhi KVK-Delhi Sh. Rakesh Kumar Assistant Gardener Yes Yes Yes 11 2 KVK Jaipur-1 KVK-Jaipur-1 N. K. Gupta Assistant Gardener Yes Yes Yes 12 2 KVK Jodhpur KVK-Jodhpur Dr. HariDayal Assistant Gardener Yes Yes Yes 13 2 KVK Sriganganagar KVK-Sriganganagar Dr. Ravi Kumar Meena Assistant Gardener Yes Yes Yes Mr. -

Is Bt Cotton in North Cotton Zone of India Under Threat from Pink Bollworm?
Weekly Publication of Cotton Association Edited & Published by Amar Singh of India 2020-21 No. 23 17th November, 2020 Published every Tuesday Cotton Exchange Building, 2nd Floor, Cotton Green, Mumbai - 400 033 Phone: 3006 3400 Fax: 2370 0337 Email: [email protected] www.caionline.in Is Bt Cotton in North Cotton Zone of India Under Threat from Pink Bollworm? Dr. Monga is a Plant Pathologist protected from American, spotted by profession. He worked as Head of and pink bollworms. Regional Station, Sirsa, Haryana of ICAR-Central Institute for However, during 2015, severe Cotton Research for more than two damage to BG II cotton by pink decades. He also worked as Principal bollworm and yield losses were observed in many regions of Investigator of Plant Pathology under Gujrat and some parts of AP, All India Coordinated Research Maharashtra and Telangana. Project on Cotton Studies conducted at for over a decade. He ICAR-CICR showed contributed significantly that pink bollworm in strengthening the had developed research and development resistance to two Cry network for cotton crop in toxins deployed in the North Zone. BG II. (Kranthi, 2015). Again in 2017-18, Maharashtra state witnessed After the introduction of single (Cry1Ac) unprecedented attack and losses due to pink boll gene transgenic cotton in Central and South Zone worm infestation in cotton. However, in North in 2002 and in North Zone of India in 2005 and cotton zone, BG II cotton hybrids continued to subsequent approval of dual gene (Cry1Ac + remain effective against pink bollworm whereas Cry2Ac BG II) hybrids in 2006, cotton crop was the pest used to cause damage (Table 1) in non Bt Table-1 Pink Bollworm Larval recovery (%) from green bolls in non Bt cotton crop at 160 days from different locations of the North Zone S.N Locations 2016-17 2017-2018 2018-19 2019-20 1. -

RAJASTHAN UNIVERSITY of HEALTH SCIENCES Kumbha Marg, Sector-18, Pratap Nagar, Tonk Road, Jaipur -302033 Phone: 0141-2792644, 2795527 | Website
RAJASTHAN UNIVERSITY OF HEALTH SCIENCES Kumbha Marg, Sector-18, Pratap Nagar, Tonk Road, Jaipur -302033 Phone: 0141-2792644, 2795527 | Website: www.ruhsraj.org TENTATIVE SEAT MATRIX FOR B.PHARM AND D.PHARM COURSES (2017-18) Amended in compliance to Hon'ble High Court orders 1) SBCWP No. 13523/2017, dated 16.08.2017 2) SBCWP No. 13070/ 2017, dated 16.08.2017 and 3) SBCWP no. 9821/2017, dated 11.08.2017 and RUHS letter 4) no. 10368, dated 30.08.2017 5) no. 10381, dated 30.08.2017 6) no. 10387, dated 30.08.2017 Following is the tentative seat matrix for B.Pharm and D.Pharm courses for the counselling purpose for the session 2017-18. Government college College Sanctioned intake College S. No. B.Pharm D.Pharm 1 Department of Pharmacy, PHTI, SMS Medical College, JLN -- 60 Marg, Jaipur Private colleges College Sanctioned intake College S. No. B.Pharm D.Pharm 2 Akashdeep College of Pharmacy, Mansarovar, Jaipur -- 60 3 Alwar Pharmacy College, MIA, Alwar 100 50 ** ** Inserted in compliance to RUHS letter no. 10368, dated 30.08.2017 4 * Arya College of Pharmacy, Kukus, Jaipur 100* -- * Intake changed from 60 to 100 in compliance to interim order of Hon'ble High Court, Jaipur (S.B. Civil Writ Petition No. 13523 / 2017, dated 16.08.2017) subject to the final outcome of the writ petition - the college to inform the 40 students as per the order of Hon'ble High Court 5 Bharti Institute of Pharmacy Science, Nathwala, -- 60 Sriganganagar 6 CLG Pharmacy College, Sumerpur, Dist. Pali -- 60 7 G.D. -

Swami Keshwanand Rajasthan Agricultural University, Bikaner Accredited by Indian Council of Agricultural Research, New Delhi AICTE Approved | Certified ISO 9001:2015
INSTITUTE OF AGRI BUSINESS MANAGEMENT Swami Keshwanand Rajasthan Agricultural University, Bikaner Accredited by Indian Council of Agricultural Research, New Delhi AICTE Approved | Certified ISO 9001:2015 PLACEMENT BBROCHUREROCHURE 22019-200 1 9 - 2 0 Agri-Business engthening Str We give Wings to Perform Our commitment to provide professionally competent managers to agribusiness and allied sectors through focus on academic excellence along with agribusiness applications. CONTENTS Vice Chancellor's 1 Message 2 3 Invitation to 66 Recruiters Course From Director's 4-5 Structure Desk About IABM 7 8 10-11 Pedagogy Our Faculty & Mentors 9 Scholastic Ambience Esteemed Visitors 12 14-15 Clubs & 13 IABM in Action 16 Committees Timeline Project @ IABM 17 20-32 Our Recruiters Students' Profile for 18-19 Placement Corporate View & Alumni Testimonials 33-37 Students' Profile for Summer Internship How to Reach Bikaner 39 To forget how to dig the earth and tend the soil is to forget ourselves. -Mahatma Gandhi Phone : 0151-2250443, 2250488 (O) : 0151 : 2250469, 2250529 (R) Prof. R. P. Singh Fax : 0151 : 2250336 Vice-Chancellor email : [email protected] SWAMI KESHWANAND RAJASTHAN AGRICULTURAL UNIVERSITY BIKANER - 334 006 RAJASTHAN Vice-Chancellor's Message The potential of Indian agriculture in the backdrop of emerging opportunities in the globalised era is making it imperative to give agricultural production and post-harvest management a professional outlook. The agribusiness sector can act as a catalyst in providing the required impetus to make the agricultural produce globally competitive at the same time ensuring income generation for all the stakeholders involved, especially the primary producers. Thus, the need for trained manpower to manage the various dimensions of the agriculture sector is becoming explicit, and is continuously increasing. -

Reportable in the Supreme Court of India Civil Appellate Jurisdiction
1 REPORTABLE IN THE SUPREME COURT OF INDIA CIVIL APPELLATE JURISDICTION CIVIL APPEAL NO. 6842 OF 2014 (arising out of SLP(C) No.35057 of 2011) Apollo College of Veterinary Medicine … APPELLANT VERSUS Rajasthan State Veterinary Council & Ors. … RESPONDENTS With C.A.No.6851/2014 (@ SLP(C) No.9216/2012) C.A.No.6852/2014 (@ SLP(C) No.9217/2012) C.A.No.6850/2014 (@ SLP(C) No.5301/2012) C.A.No.6853/2014 (@ SLP(C) No.9766/2012) C.A.No.6854/2014 (@ SLP(C) No.13493/2012) C.A.No.6857/2014 (@ SLP(C) No.23247/2012) C.A.Nos.6844-6845/2014 (@ SLP(C) Nos.142-143/2012) C.A.Nos._6846-6849/2014 (@ SLP(C) Nos.1900-1903/2012) C.A.No.6855/2014 (@ SLP(C) No.20779/2012) C.A.No.6856/2014 (@ SLP(C) No.22303/2012) J U D G M E N T SUDHANSU JYOTI MUKHOPADHAYA,J. Leave granted. 2. As all these appeals involve the same issue relating to the recognition of Veterinary College(s) under Veterinary Council of India Act, 1984; they were heard together and are disposed of by this common judgment. 3. Apollo College of Veterinary Medicine, Jaipur (for short “Apollo College”) and Mahatama Gandhi College, Bharatpur (for short “Mahatama Gandhi College”) are two private colleges in Page 1 2 Rajasthan offering the course of Bachelor of Veterinary Science and Animal Husbandry (hereinafter referred to as “B.V.Sc. & A.H”). A number of students of the said colleges have passed the B.V.Sc. & A.H examination conducted by the Rajasthan Agricultural University (now known as Swami Keshwanand Rajasthan Agricultural University, Bikaner), recognized by Veterinary Council of India and Central Government. -

Fertility Status of Primary Nutrient in Kinnow Orchard in Irrigated Area of Sriganganagar District of Rajasthan” Was Under Taken During April 2016 to April 2017
Journal of Pharmacognosy and Phytochemistry 2018; 7(4): 1615-1619 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2018; 7(4): 1615-1619 Fertility status of primary nutrient in Kinnow Received: 16-05-2018 Accepted: 18-06-2018 orchard in irrigated area of Sriganganagar district of Rajasthan JS Shekhawat Department of Horticulture, College of Agriculture Bikaner, Swami Keshwanand Rajasthan JS Shekhawat, IM Verma, PK Yadav and RK Narolia Agriculture University Bikaner, Rajasthan, India Abstract The experiment was conducted on “Fertility status of primary nutrient in kinnow orchard in irrigated area IM Verma of sriganganagar district of rajasthan” during April, 2016 to April, 2017. The ninety soil samples with Department of Horticulture, three depths i.e., 0-30, 30-60 and 60-90 cm were collected from thirty kinnow orchards from different College of Agriculture Bikaner, Swami Keshwanand Rajasthan five tehsil (suratgarh, Raisinghnagar,sri vijaynagar, sri karanpur And sriganaganagar)of sriganganagar Agriculture University Bikaner, district. The soil samples were analyzed for nutritional status of kinnow orchards being grown at farmers Rajasthan, India field. The kinnow orchard soils in this investigation were found low in organic carbon and available Nitrogen, medium in available phosphorus, medium to high in available potassium low to high in PK Yadav available, respectively. Department of Horticulture, College of Agriculture Bikaner, Keywords: Fertility status primary nutrient Kinnow orchard irrigated area Sriganganagar Swami Keshwanand Rajasthan Agriculture University Bikaner Introduction Rajasthan, India India has large arid zones covering an area of 317090 sq km mainly located in the North-West RK Narolia parts of the country Rajasthan alone covers 62 per cent area under arid zone. -

Testing Centers.Pdf
Annexure-V List of institutions approved by the Department of Agriculture & Cooperation, Ministry of Agriculture, Government of India for Testing and Certifying Agricultural Machineries and Equipments S. Name of the State Name of the Institute No. Acharya N.G. Ranga Agriculture University (ANGRAU), Rajendra 1 ANDHRA PRADESH Nagar, HYDERABAD (ANDHRA PRADESH) Faculty of Agricultural Engineering, Rajendra Agriculture 2 BIHAR University, PUSA (BIHAR) State level Agriculture Implement Testing Centre, Directorate CHHATTISGARH 3 (Agricultural Engineering), Agriculture Department, Govt. of Chhattisgarh, TeliBandha, Gorav Path, RAIPUR (CHHATTISGARH) Division of Agricultural Engineering, Indian Agricultural Research 4 DELHI Institute, NEW DELHI-12. College of Agricultural Engineering & Technology, Junagarh 5 GUJARAT Agricultural University, JUNAGARH (GUJARAT) College of Agricultural Engineering & Technology, 6 HARYANA ChoudharyCharan Singh Agriculture University, HISSAR (HARYANA) Sher-e-Kashmir University of Agri. Science & Technology, 7 JAMMU & KASHM IR SRINAGAR REGION (J. & K.) and JAMM U REGION (J. & K.) 8 Birsa Agriculture University, Kanke, RANCHI (JHARKHAND) Jharkhand Agriculture Machinery Testing and Training Centre (JAM-TTC), Department of Agriculture and Cane Development JHARKHAND 9 (Directorate of Soil Conservation), Jharkhand, Agricultural Engineering Section at Extension Training Campus, Govt. of Jharkand, RANCHI (JHARKHAND) University of Agricultural Sciences, Gandhi KrishiVignyan Kendra, 10 KARNATAKA BANGALORE (KARNATAKA.) 11 College -

5Th National Seminar on Climate Resilient Saline Agriculture
5th National Seminar Advisory Committee First Announcement Climate Resilient Saline Agriculture : Sustaining Livelihood Security Chairman Date: 21-23 January 2017 Dr. Trilochan Mohapatra, Secretary, DARE and Director General, ICAR, New Delhi th Members 5 National Seminar Organised by Dr. Gurbachan Singh, Chairman, ASRB, New Delhi Indian Society of Soil Salinity and Water Quality, Karnal (Haryana) Dr. K. Alagusundaram, Deputy Director General (A), NRM, ICAR, New Delhi on S. K. Rajasthan Agricultural University, Bikaner (Rajasthan) Dr. B.R. Chhipa, Vice Chancellor, S.K. Rajasthan Agricultural University, Bikaner (Rajasthan) ICAR-Central Soil Salinity Research Institute, Karnal (Haryana) Dr. D.R. Bhumbla, Former DDG (SAE), ICAR, & Former Director, ICAR-CSSRI, Karnal (Haryana) Climate Resilient Saline Agriculture: REGISTRATION FORM Dr. I.P. Abrol, Former DDG (SAE), ICAR, & Former Director, ICAR-CSSRI, Karnal (Haryana) Dr. N.T. Singh , Former Director, ICAR-CSSRI, Karnal (Haryana) Sustaining Livelihood Security Name (Block letters) ___________________________________ Dr. N.K. Tyagi, Former Member, ASRB, and Former Director, ICAR-CSSRI, Karnal (Haryana) Profession ___________________ Designation _____________ Dr. B. Mishra, Chairman (Research Advisory Committee, ICAR-CSSRI) 21–23 January 2017 Organization ________________________________________ Dr. D.K. Sharma, Former Director, ICAR-CSSRI, Karnal (Haryana) and President, ISSSWQ Mailing Address ______________________________________ Dr. P.C. Sharma, Director (A), ICAR-CSSRI, Karnal (Haryana) -

1. Mr Anooj S S Division of Entomology ICAR-Indian
List of Members declared as Life Fellows of the Entomological Society of India (2020) 1. Mr Anooj S S 14. Dr O. Shaila Division of Entomology Regional Agricultural Research Station (Professor ICAR-Indian Agricultural Research Institute New Delhi Jayashankar Telangana State Agricultural University, 110012 Hyderabad), Palem 509215, Nagar Kurnool District, Telangana 2. Dr. Anil Kumar Dubey Zoological Survey of India 15. Mr S. Srinivasnaik Andaman and Nicobar Regional Station Port Blair 744102, Department of Entomology, Agricultural College, Jagtial A&N Islands (Prof JTSAU, Hyderabad), Polasa, Jagtial 505529, Telangana 3. Ms Swati G. Lonagre De partment of Entomology 16. M r. Thammali Hemadri Dr Panjabrao Deshmukh Krishi Vidyapeeth, Akola Department of Agricultural Entomology University of 44 4104, Maharashtra Agricultural Sciences Raichur 584102, Karnataka 4. Dr Prashant Wasudeorao Nemade Cotton Research Unit 17. M r. Rudra Narayan Borkakati AICRP on Biological Dr Panjabrao Deshmukh Krishi Vidyapeeth Akola 444104, Control Maharashtra Department of Entomology, Assam Agricultural University, 5. Dr. Rahul Murar Wadaskar Jorhat 785013, Assam Department of Entomology, (Dr PDKV) College of Agriculture, Nagpur 440001, Maharashtra 18. Dr (Mrs) Neelam Yadav Post Doctoral Fellow (UGC) 6. Dr Jayashri D Ughade Chandra Shekhar Azad University of Agriculture and Department of Entomology, College of Agriculture Technology (Dr Panjabrao Deshmukh Krishi Vidyapeeth) Nagpur Kanpur 208002 Uttar Pradesh 440001 Maharashtra 19. Dr K. Ravi Kumar 7. Dr. Bhamare Vijay Krishnarao Krishi Vigyan Kendra, Wyra (Professor Jayashankar Department of Entomology, College of Telangana State Agricultural University, Hyderabad), Agriculture, (Vasantrao Naik Marathwada Krishi Khammam 507165, Telangana Vidyapeeth, Parbhani) Latur 413512, Maharashtra 20. Dr Pavani Thatikonda 8. Ms Sunitha Pola Department of Entomology, Agricultural College Horticultural Research Station (Professor Jayashankar Telangana State Agricultural Dr. -

Status for the AY 2015-16 As on March 2016.Xlsx
Status of Release of Scholarship to Students Under J&K SSS - 2015-16 (Till 31.03.2016) Note: 1. For any query sent mail to [email protected] 2. Scholarships shown as released in AY 2015-16 are subject to veification and confirmation of account details of students for DBT Admission Course S.No. Candidate Name Through College Name State Reason Category ID Counseling 1 20177688 Aijaz Ahmad Wani Yes Aditya College of Engineering Andhra Pradesh Engineering Claim Not Received 2 20173624 Muzafar Gull Yes Aditya Engineering College Andhra Pradesh Engineering Claim Not Received 3 20163362 Tufail Ahmad Khan Yes Aditya Engineering College Andhra Pradesh Engineering Claim Not Received Andhra University College Of 4 20181615 Aatika Anjum Yes Andhra Pradesh Engineering Claim Not Received Engineering Andhra University College Of 5 20163462 Danish Amin Lone Yes Andhra Pradesh Engineering Claim Not Received Engineering Global College Of Engineering & 6 20150954 Asif Mushtaq Yes Andhra Pradesh Engineering Claim Not Received Technology Jntua College Of Engineering 7 20168013 Nissar Ahmad Ganie Yes Andhra Pradesh Engineering Released Pulivendula 8 20176795 Ruqiyah Aslam Yes Kaushik College Of Engineering Andhra Pradesh Engineering Claim Not Received MVR College Of Engineering 9 20170197 Deepak Gupta Yes Andhra Pradesh Engineering Claim Not Received And Technology Praveenya Institute Of Marine 10 20169472 Kaisir Ashraf Yes Andhra Pradesh Engineering Claim Not Received Engineering & Maritime Studies Sri Krishnadevaraya University 11 20178307 -

College of Home Science Bikaner SYLLABUS 2019-20
College of Home Science Bikaner SYLLABUS 2019-20 B.Sc. (Hons.) CommunityScience (4 Years Programme) COLLEGE OF HOME SCIENCE, BIKANER SWAMI KESHWANAND RAJASHTHAN AGRICULTURAL UNIVERSITY BIKANER-334006 1 SYLLABUS 2019-20 COLLEGE OF HOME SCIENCE SWAMI KESHWANAND RAJASHTHAN AGRICULTRAL UNIVERSITY BIKANER-334006 2 Compiled & Edited by : Dr. SUNITA LADDHA Published by : College of Home Science Swami Keshwanand Rajasthan Agricultural University Bikaner-33 4006 3 PREFACE The education division of Indian Council of Agriculture Research (ICAR) felt need to make some changes in degree and syllabus as per the present need . Council (ICAR) constituted Vth Deans Committee for the quality improvement in education as well as to consider the contemporary challenges for employability of passing out graduates , lots of efforts have been made to improve the quality of education ,to make it internationally competitive . The implementation of the recommendation of Vth Deans Committee have made mandatory for all the SAUs. Therefore the nomenclature of degree has been changed from Home Science to Community Science and introduces one more new course named Food Nutrition and Dietetics has been introduced under the umbrella of Home Science . Further from the year 2017-18 college of Home Science (Community Science) will offer two degrees namely B.Sc. (Hons) Community Science and B.Sc. (Hons) Food Nutrition and Dietetics. Therefore the required modifications were incorporated in new syllabus was approved in 49 Academic Council Meeting held on 25.06.2017 as well as by the 9th Board of Studies (2017) . This syllabus is being distributed among all the concerned students with the hope that it will be of great use for all the students to achieve objective of each subject in their course curriculum.