Head Lice Treatment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Comparison Chart
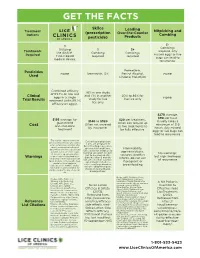
GET THE FACTS Sklice Leading Treatment Nitpicking and (prescription Over-the-Counter Options Combing pesticide) Products 3+ 1 Combings Utilizing 1 3+ Treatments required. Any the AirAllé® Combing Combings missed eggs or live Required FDA-cleared required required bugs can lead to medical device. recurrence. Permethrin, Pesticides none Ivermectin, .5% Benzyl Alcohol, none Used Lindane, Malathion 76% in one study, of 93.7% on lice and and 71% in another 20% to 86% for Clinical eggs in a single study for live live lice only none Trial Results treatment (with 99.2% lice only $270 average. $90 per hour. $195 average, for $20 per treatment. $140 to $320 Usually takes a guaranteed Often can require up Often not covered minumum of 3-5 Cost one-and-done by insurance. hours. Any missed treatment be fully eective. eggs or live bugs can lead to recurrence. ® The AirAllé device cannot be Consult your physician used to treat persons who cannot if you are pregnant or sense temperature or pain; who breastfeeding, have aller- cannot communicate physical gies or skin conditions. Flammability, discomfort; who have open head Can cause eye irritation or wounds, sores, or visible signs age restrictions, burning sensation on skin. No warnings; of skin or scalp abnormalities Not to be used on chil- seizures, death in or who have received radiation but high likelihood Warnings dren less than 6 months infants, do not use treatment of the head within the old. Eects of an overdos- of recurrence last 6 months; or those who have age include rash, dizzi- if pregnant or cranial or facial implants. -

EPI Update Sept 2010-Head Lice.Pmd
September 2010 Epidemiology Update Head Lice Introduction Head Lice Key Points This Epidemiology Update presents information about head lice. This issue is different in that it does not present data. Past issues profile disease data in Hennepin County. This issue of ♦ Head lice are primarily transmitted by Epidemiology Update is one in a series of reports from direct head-to-head contact. Sharing Hennepin County Human Services and Public Health personal items, such as hats, combs, Department—Epidemiology available at: and sports head gear can also transmit www.hennepin.us/EpiUpdates head lice. Background ♦ The head louse (Pediculus humanus capitis) is an obligate Head lice do not jump or fly; they parasite of humans found on the scalp. The head louse’s body is crawl and can fall off the head. Head less than 1/8 inch in length and grayish white or tan in color. lice do not live longer than 48 hours off Female lice live for 3 to 4 weeks and lay up to 10 nits (eggs) per the head and lice eggs (nits) cannot day. Nits are very small (about the size of the eye of a small hatch at an ambient temperature lower needle), gray or white in color and are attached near the base of than that near the scalp. Regular the hair shaft. The most viable nits are found within 1/4 inch of cleaning and laundering of clothing and the scalp. Nymphal head lice emerge from the nits after 7 to 10 bedding are the only measures needed days. Nits found more than 1/2 inch from the scalp are dead or to rid the environment of head lice. -

Managing Head Lice in Schools
Managing Head Lice in Schools Center of Expertise for School IPM School IPM Refresher Integrated Pest Management (IPM) is a smarter, usually less costly option for effective pest control in the school community. An IPM program employs common sense strategies to reduce sources of food, water and shelter for pests in your school buildings and grounds. IPM programs take advantage of all pest management strategies, including the judicious use of pesticides. Center of Expertise for School IPM IPM Basics Pesticides Physical & Mechanical Controls Cultural & Sanitation Practices Education & Communication Center of Expertise for School IPM Key Concepts Inspect and monitor for pests and pest conducive conditions Prevent and avoid pests through exclusion and sanitation Use treatments that minimize impacts on health and the environment Everyone has a role - custodians, teachers, students, principals, and pest management professionals Center of Expertise for School IPM Benefits of School IPM Smart: addresses the root cause of pest problems Sensible: provides a healthier learning environment Sustainable: better long-term control of pests Center of Expertise for School IPM Presenters Richard Pollack, Ph.D. • Senior Environmental Public Health Officer, Harvard University • Public Health Entomologist, Harvard School of Public Health • Chief Scientific Officer, IdentifyUS • International expert, presenter and author on medically relevant pests Nichole Bobo, MSN, RN • Nursing Education Director, National Assoc. of School Nurses • Oversight of NASN -

Head Lice Treatment: Heading Off an Ancient Adversary
Head Lice Treatment: Heading Off an Ancient Adversary Wendy L. Wright MS, RN, APRN, FNP, FAANP, FAAN American School @ASHAnews Health Association About ASHA • The only national organization dedicated to a coordinated, multidisciplinary approach to school health • ASHA membership • Administrators in health and education agencies at the local, state and federal levels • Health and education professionals in the PreK-12 school setting • Counselors, dieticians, nutritionists, health educators, physical educators, psychologists, school health coordinators, school nurses, school physicians, and social workers • Academics who conduct research that informs school health professionals • Journal of School Health (JOSH) Membership Benefits- Professional Level • 12 print and online issues of the Journal of School Health per year • Access to the School Health Listserv (CHEN) • Access to the weekly School Health Action e-newsletter • Discounts on the Annual School Health Conference • Free Continuing Education contact hours • Leadership opportunities to serve on the national board or as committee chairperson • Engagement opportunities to serve on various national committees www.ashaweb.org American School @ASHAnews Health Association Save the Date! • Administration, Coordination, and Leadership • Programs and Services • Research and Emerging Issues • Teaching and Learning The WSCC Framework The Whole School, Whole Community, Whole Child (WSCC) model requires us to take a systems-based approach to health promotion. Only when all members of the school and community work together can we address problems. Head Lice Treatment: Heading Off an Ancient Adversary Wendy L. Wright MS, RN, APRN, FNP, FAANP, FAAN American School @ASHAnews Health Association Centers for Disease Control and Prevention (CDC). Head Lice Treatment Heading Off an Ancient Adversary PP-SKL-US-0103 5/16 Presentation Outline I. -

Head Lice Management in the School Environment
Head Lice Management in the School Environment Lice are parasites that can be found on people's heads and bodies. Lice move by crawling; they cannot hop or fly. Head lice problems occur year-round. However, it is during the school year that head lice cases seem to be the most common. The reason is that many head louse infestations are discovered by teachers and school nurses. Children are usually sent home, and their parents are notified. It is the school nurse’s responsibility to manage head lice problems in the school setting (through head checks, child exclusion, etc.), and it is the parents responsibility to control head lice in their own children. Prevention of Head Lice in Schools Head lice are spread from person-to-person through close body contact, or through sharing combs, brushes, hats, and other head gear. Lice do not infest classrooms, carpets, and chairs. Person-to-person spread can be minimized in the school environment by doing the following: (1) Space desks and chairs apart so that children are not sitting shoulder-to-shoulder. (2) Have children hang coats and hats separately. Do not pile them on top of each other. (3) Space children apart when standing or walking in lines. (4) During head lice outbreaks, minimize close contact games and sports, such as wrestling. (5) During outbreaks, minimize use of shared head-gear (such as earphones, helmets, etc.) and clothing (such as hats and costumes in drama classes). Always hand-vacuum head-gear between users. (6) Provide head louse prevention education to children, such as not sharing combs, brushes, hats, headbands, or clothing. -

Headlice/Nits
VAN INDEPENDENT SCHOOL DISTRICT Lice Prevention, Control, and Treatment Protocol Policy Based on recommendations from the American Academy of Pediatrics, 2010, and the 2013 update to the Texas Administrative Code (TAC Title 25, part 1, Chapter 97, Subchapter A, Rule 97.7), Van ISD has updated its Lice Prevention, Control, and Treatment Protocol to reflect the most current guidelines in the control of lice. Students should not be excluded from school due to head lice. The Centers for Disease Control and Prevention (CDC), the American Academy of Pediatrics (AAP), and the Texas Department of Health Services (DSHS) support that there is little evidence that exclusion from school reduces the transmission of head lice (DSHS, 2007). Head lice are common for children ages 3-12. Head lice are not a health hazard and are not responsible for the spread of any disease. They are the cause of much embarrassment, misunderstanding, and many unnecessary days lost from work and school. “No-nit” policies which keep kids with lice home as long as they have any evidence of an infestation do not benefit students or their classmates and “should be abandoned” (AAP, 2010). The goal of lice prevention, control, and treatment in schools is to prevent the spread of lice between students. Lice control involves a joint effort between the home, school, and after-school programs. Questions regarding Van ISD Lice Policy, Protocol, and Procedures should be directed to the campus nurse or the administration. Initial Identification of Infestation Cases of lice should be confirmed by the school nurse. Confidentiality will be maintained and information about any student with lice or nits will not be shared with other students or staff members. -

The Best Control for Lice and Scabies
OUCH! BITING, STINGING AND/OR BLOOD FEEDERS OVERVIEW Have You Just Been Bitten or Stung? What you don’t know can hurt you. When it comes to bites and stings - prevention is your best medicine. What can you do? First, learn which creatures can bite or sting. Next, understand what you can do to avoid a problem. Last, know what to do and whom to call if you get bitten or stung. Help is simply a phone call away. If you are bitten or stung, call your local health care provider or physician to determine if you can be treated at home, need to be seen by a physician or should go directly to a hospital emergency department. If you can be treated at home, your health care provider should keep in phone contact with you by calling back and checking on your condition. If your condition worsens, you should go to the nearest emergency department or call the paramedics or an ambulance. The following suggestions are not intended to replace proper medical care. Always treat a small area before using any product, herb, oil, shampoo, soap, cleaner or any material you put on your body. Things you can do to prevent venomous bites or stings: (It helps if you do not look or smell like a flower or wear flashy jewelry.) When hiking - ☺ Wear plain, light-colored clothing: long, heavy ☺ Don’t wave at, yell at, handle, annoy, touch or pants, long sleeves, netting and high-top, lace-up try to play with wild animals, spiders, stinging leather shoes or boots that cover the ankles. -

Cooties Defined by RICHARD J
18 Winter 2015 Applegater Notes from a Rogue entomologist Cooties defined BY RICHARD J. HILTON As another election year draws rather appalled by this near, it seems appropriate to write about occurrence, I will freely blood-sucking parasites. admit that I did find it I am old enough to remember somewhat fascinating. when the playground taunt “be careful The fact that adults or you’ll get cooties” was commonplace. do not commonly get We even had the game “Cootie,” where infested by head lice the goal was to construct an insect model. may have played a role However, growing up I really had no in my dispassionate idea what a cootie was. I thought it was approach to this a reference to germs in general and not to problem. This event did lice, the wingless blood-sucking insects. take place almost 20 Head louse image from a scanning electron microscope And due to my sheltered life as a youth years ago, and I recall (www.dailymail.co.uk/sciencetech/article-2480876/). and the widespread use of insecticides for that it was one of the louse control after World War II, I never first times that I really had a close encounter with lice when I used the Internet to was in school. So it was a bit startling research a topic, finding when our daughter came down with a that, while it was an case of head lice while at Jacksonville incredible source of Elementary School. information, it was also These insects are obligate full of bogus remedies. ectoparasites, meaning they have to In other words, not be living on a host. -

Bug of the Month
A topical review of infection-related issues Nitpicking the Details About Lice Jared Bullard, MD; and John M. Embil, MD, FRCPC Very few things will give people a peculiar itchy feeling more than the mentioning of lice. Pediculosis is a common infestation, particularly in the pediatric population. The three species of lice ( i.e. , Pediculus humanus corporis , Pediculus humanus capitis and Phthirus pubis) account for most of the cases seen in practice; therefore, they are this month’s Bug of the Month . What is pediculosis? of clothing and infesting other parts of the combs to hair elastics. Girls and those of body. P. pubis , which does resemble a crab, European ancestry are more likely to be Pediculosis refers to an infestation with one prefers the coarse hair typical of the groin infested. of three species of lice ( i.e. , P. humanus region but can be found in other locales, P. corporis is seen in situations of poor © ion corporis , P. humanus capitis and P. pubis ). such as body or axillary hair hor evten on the sanitation, orveirbcrowudintg and in the home - Each has a predilection for specific areas of eyebrows and eyelasheis.g less.iOsutbtreaks aaredty,pically seen in times yr D wnlo the body and causes variable amounts of p ial of wnardanod decreased personal hygiene and o rc s ca se pruritis and rash as a result of injection of C e user are spnreadl uby those with endemic lice or What is the lmife cyiscelde erso saliva and a secondary hypersensitivity om thor for p “lousy” people. -

Dermatologic Infestations
DERMATOLOGIC INFESTATIONS Roberta C. Romero, M.D. Tropical Disease Foundation INFESTATIONS Definition: To inhabit (sometimes in quantities large enough) to be harmful, threatening or obnoxious INFESTATIONS CREEPING ERUPTIONS SCABIES LICE – HEAD, BODY, AND PUBIC LICE CREEPING ERUPTIONS CREEPING ERUPTIONS (LARVA MIGRANS) Infestation with the larvae of a cat or dog hookworm-Ancylostoma braziliensi or caninum Usual scenario: cat or dog infected with hookworm lays down eggs on soil larvae hatch from eggs penetrate skin Larva cannot go beyond basement membrane moves underneath skin CREEPING ERUPTIONS Recognition: Serpiginous erythematous rash Lesions may have papules and vesicles Very pruritic Complication: Secondary bacterial infection CREEPING ERUPTIONS Treatment: Oral: Thiabendazole 50 mgs/kg/day x 5days Albendazole Ivermectin Freeze with liquid nitrogen Topical: make a thiabendazole ointment by crushing tablets and mixing with petrolatum S C A B I E S SCABIES - HISTORY From Latin word “scabere” = to scratch Deuteronomy 28:27 – first record (festering sores and rash with incurable itch) Aristotle – lice that escaped from pimples (315BC) Celsus – described in detail clinical picture (30 AD) Giovanni Cosimo Bonomo (1687) – “Obervations concerning the fleshworm of the body” causing severe itch. INFESTATIONS - SCABIES Highly infectious disease caused by Sarcoptes scabeie var. humanus Usual scenario: An infected person (friend, kin, classmate, lover, Inday, with mature adult female mite) comes in close contact mite transfers (sometimes sharing -

Live Lice Free-The Headlice Handbook
Live Lice Free The Headlice Handbook Tips, tricks, articles and remedies so you too can beat the little nasties forever! Live Lice Free The Headlice Handbook Tips, Tricks, Articles and Safe, Natural Remedies. Table of Contents Introduction ................................................................................................ 3 Head Lice information and FAQ ............................................................. 7 What Do Head Lice Look Like?................................................................ 9 Head Lice Home Remedies? But Don’t PEOPLE Get Head Lice?.... 12 The Things Chemical Head Lice Product Manufacturers Don’t Want You to Know................................................................................... 16 Eliminate Lice Safely – How to Choose a Head Lice Treatment. ..... 21 Does Mayonnaise Treat Head lice? 3 Alternative Tips....................... 25 Method One - Essential Oils - Our Head Lice Recipe for Success.... 27 ZAP! Our Head Lice Remedy ................................................................. 30 Method Two - Heat ................................................................................. 35 The Essential Oil Home Medicine Chest – The Oils.............................. 36 Safe Remedy For Pregnancy ................................................................. 42 More Safe Home Remedies ................................................................... 44 Proceed With Caution. ........................................................................... 61 Do Not Pass Go. ...................................................................................... -

Medwatch Report
Triage Unit Sequence # MedWatch A. Patient Information C. Suspect medication(s) Patient Identifier Date of birth Sex Weight Name: lindane 1435 05/04/1950 male 160 lbs B. Adverse event or product problem Dose, frequency, route use Therapy dates Product Problem 5% 04/2004 to Outcomes attributed to adverse event 01/2005 death disability Diagnosis for use Event abated after use life-threatening congenital anomaly Prescription was for two (2) stopped or dose reduced hospitalization required intervention uses/treatments. no other: Lot # Exp. date Event reappeared after Date of event 04/20/04 Date of report 1/26/2005 Anti-biotics reintroduction Describe event or problem yes NDC # - - In April of 2004 I became infested with lice from a visit to the hospital. Concomitant medical products Ive tried mayonaise, over-the-counter shampoos, "internet- ordered" shampoos/treatments, essential oils, etc. I have quite a collection of lice and nit removal combs whick I have D. Suspect medical device Brand name Type of device Manufacturer name and address Operator of device health professional user facility distributor Expiration date model # _____________________ Relevant tests/laboratory data catalog # ____________________ If implanted, give date serial # _____________________ lot # ________________________ If explanted, give date other # _____________________ Device available for evaluation? yes no returned to manufacturer __/__/____ Concomitant medical products Other relevant history, including preexisting condition I've got itchy "crawly-feeling" scalp. I have NOT been able to locate any actual lice and nits; however, I wash out a E. Reporter number of "questionable" things out of my hair on a regular Name and address phone # (781)449-6487 basis.