Guidance About the FFCRA, the CARES Act, and Other Health Coverage Issues Related to COVID-19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spring Announcements March 18, 2021
Parks and Recreation Announcements Updated March 18, 2021 The City of Duluth Parks and Recreation Division announces the following updates to recreational amenities. The announcements are released in conjunction with the updated Parks and Recreation Pandemic Operations Plans Policy. Parks and Recreation offerings and maintenance for Spring and Summer 2021 strive to align as much as possible with allowable activities defined by State guidelines: https://mn.gov/covid19/for-minnesotans/stay-safe-mn/stay-safe-plan.jsp The updated Parks and Recreation Pandemic Operations Plans Policy applies to park and facility use scheduled on or after April 1, 2021 and can be found at: https://duluthmn.gov/parks/covid/ A. Permits and Reservations Parks and Recreation is currently accepting permit requests for outdoor activities, reservations, and events at parks, trails, pavilions, etc. Proposed activities must comply with State guidelines in effect at the time of the activity. The Parks and Recreation Pandemic Operations Plans policy will apply to all permits and reservations. B. Spring 2021 City-owned buildings updates Parks and Recreation, in collaboration with Property and Facilities Management, has identified a set of buildings to reopen for use by the public through our standard reservation process. Selection of these buildings is based on previous reservation and use patterns, staff capacity for maintenance of facilities, and occupancy limitations on indoor gathering sizes. The Parks and Recreation Pandemic Operations Plans policy will apply to all permits and reservations. Now accepting reservations: Washington Center Gym Evergreen Senior Center Morgan Park Community Center Wade Stadium Accepting reservations for dates May 15 and beyond: Lafayette Community Center Park Point Beach House Other City-owned facilities that are occupied by tenant-lease agreements continue to operate under the terms of their lease agreements and approved pandemic safety plans. -

FAQ Travel Restrictions (Updated March 18 2020)
FAQ – TRAVEL RESTRICTIONS (As of March 18, 2020, 1:30 p.m.) Q1. What is being done to ensure our service members are safe in their travels and keep them healthy? A1. The actions outlined in Secretary Esper’s memo and the Force Health Protection Supplement #4 are done to protect our personnel and their families. The department will take several measures to enhance traveler safety, such as establishing pre- and post-travel screening and reception procedures and transitioning to military and DoD contracted aircraft for from or to CDC Level 3 or Level 2 designated areas. Additionally, DoD has instituted domestic official travel restrictions through May 11 (e.g. Permanent Change of Station and Temporary Duty) to safeguard our people. We encourage all DoD personnel and families to visit https://www.defense.gov/Explore/Spotlight/Coronavirus/ for tips on staying healthy. Q2. Why did DoD institute travel restrictions on its people? A2. In order to help limit COVID-19’s spread and its impact on the force, the Secretary of Defense instituted travel restrictions for both international and domestic travel. Q3. Who does the domestic travel restriction apply to? A3. All DoD service members and civilians, and their family members will stop all official travel – such as Permanent Change of Station or Temporary Duty – through May 11. Exceptions may be given for compelling cases where the travel is: (1) determined to be mission essential; (2) necessary for humanitarian reasons; or (3) warranted due to extreme hardship. Q4. When does the domestic travel restriction go into effect? A4. March 16. -

Regarding Private Flood Insurance’’ on Adopted Floodplain Management ZA16 for This Rulemaking
14696 Federal Register / Vol. 86, No. 51 / Thursday, March 18, 2021 / Proposed Rules For the Nuclear Regulatory Commission. respective flood insurance regulations. • Viewing Comments Electronically— Kevin A. Coyne, The Agencies solicit comment on all Regulations.gov: Go to https:// Acting Director, Division of Rulemaking, aspects of these new questions and regulations.gov/. Enter ‘‘Docket ID OCC– Environmental, and Financial Support, Office answers. 2020–0033’’ in the Search Box and click of Nuclear Material Safety and Safeguards. ‘‘Search.’’ Click on the ‘‘Documents’’ tab DATES: Comments on the proposed [FR Doc. 2021–05570 Filed 3–17–21; 8:45 am] and then the document’s title. After questions and answers must be clicking the document’s title, click the BILLING CODE 7590–01–P submitted on or before May 17, 2021. ‘‘Browse Comments’’ tab. Comments can ADDRESSES: Interested parties are be viewed and filtered by clicking on DEPARTMENT OF THE TREASURY invited to submit written comments to: the ‘‘Sort By’’ drop-down on the right OCC: Commenters are encouraged to side of the screen or the ‘‘Refine Office of the Comptroller of the submit comments through the Federal Results’’ options on the left side of the Currency eRulemaking Portal. Please use the title screen. Supporting materials can be ‘‘Loans in Areas Having Special Flood viewed by clicking on the ‘‘Documents’’ 12 CFR Part 22 Hazards; Interagency Questions and tab and filtered by clicking on the ‘‘Sort Answers Regarding Private Flood By’’ drop-down on the right side of the [Docket ID OCC–2020–0033] Insurance’’ to facilitate the organization screen or the ‘‘Refine Documents FEDERAL RESERVE SYSTEM and distribution of the comments. -

Early Dance Division Calendar 17-18
Early Dance Division 2017-2018 Session 1 September 9 – November 3 Monday Classes Tuesday Classes September 11 Class September 12 Class September 18 Class September 19 Class September 25 Class September 26 Class October 2 Class October 3 Class October 9 Class October 10 Class October 16 Class October 17 Class October 23 Class October 24 Class October 30 Last Class October 31 Last Class Wednesday Classes Thursday Classes September 13 Class September 14 Class September 20 Class September 21* Class September 27 Class September 28 Class October 4 Class October 5 Class October 11 Class October 12 Class October 18 Class October 19 Class October 25 Class October 26 Class November 1 Last Class November 2 Last Class Saturday Classes Sunday Classes September 9 Class September 10 Class September 16 Class September 17 Class September 23 Class September 24 Class September 30* Class October 1 Class October 7 Class October 8 Class October 14 Class October 15 Class October 21 Class October 22 Class October 28 Last Class October 29 Last Class *Absences due to the holiday will be granted an additional make-up class. Early Dance Division 2017-2018 Session 2 November 4 – January 22 Monday Classes Tuesday Classes November 6 Class November 7 Class November 13 Class November 14 Class November 20 No Class November 21 No Class November 27 Class November 28 Class December 4 Class December 5 Class December 11 Class December 12 Class December 18 Class December 19 Class December 25 No Class December 26 No Class January 1 No Class January 2 No Class January 8 Class -
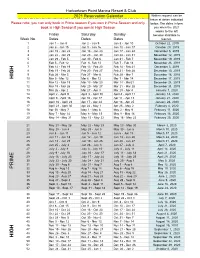
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

9/11 Report”), July 2, 2004, Pp
Final FM.1pp 7/17/04 5:25 PM Page i THE 9/11 COMMISSION REPORT Final FM.1pp 7/17/04 5:25 PM Page v CONTENTS List of Illustrations and Tables ix Member List xi Staff List xiii–xiv Preface xv 1. “WE HAVE SOME PLANES” 1 1.1 Inside the Four Flights 1 1.2 Improvising a Homeland Defense 14 1.3 National Crisis Management 35 2. THE FOUNDATION OF THE NEW TERRORISM 47 2.1 A Declaration of War 47 2.2 Bin Ladin’s Appeal in the Islamic World 48 2.3 The Rise of Bin Ladin and al Qaeda (1988–1992) 55 2.4 Building an Organization, Declaring War on the United States (1992–1996) 59 2.5 Al Qaeda’s Renewal in Afghanistan (1996–1998) 63 3. COUNTERTERRORISM EVOLVES 71 3.1 From the Old Terrorism to the New: The First World Trade Center Bombing 71 3.2 Adaptation—and Nonadaptation— ...in the Law Enforcement Community 73 3.3 . and in the Federal Aviation Administration 82 3.4 . and in the Intelligence Community 86 v Final FM.1pp 7/17/04 5:25 PM Page vi 3.5 . and in the State Department and the Defense Department 93 3.6 . and in the White House 98 3.7 . and in the Congress 102 4. RESPONSES TO AL QAEDA’S INITIAL ASSAULTS 108 4.1 Before the Bombings in Kenya and Tanzania 108 4.2 Crisis:August 1998 115 4.3 Diplomacy 121 4.4 Covert Action 126 4.5 Searching for Fresh Options 134 5. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

2021-2022 Academic Calendar
2021-2022 ACADEMIC CALENDAR QUARTER TERMS* Term ID Class Start Date Class End Date Holiday/Breaks WINTER 2021 Winter January 11, 2021 April 2, 2021 January 18, 2021 • Martin Luther King Jr. Day, No Classes April 4-11, 2021 • Spring Break, No Classes SPRING 2021 Spring April 12, 2021 July 2, 2021 May 31, 2021 • Memorial Day, College Closed July 4-11, 2021 • Summer Break, No Classes July 5, 2021 • Independence Day Observed, College Closed SUMMER 2021 Summer July 12, 2021 October 1, 2021 September 6, 2021 • Labor Day, College Closed FALL 2021 Fall October 4, 2021 December 23, 2021 November 25-26, 2021 • Thanksgiving, College Closed WINTER 2022 Winter January 10, 2022 April 1, 2022 January 17, 2022 • Martin Luther King Jr. Day, College Closed April 2-10, 2022 • Spring Break, No Classes SPRING 2022 Spring April 11, 2022 July 1, 2022 May 30, 2022 • Memorial Day, College Closed June 20, 2022 • Juneteenth Day, College Closed July 2-10, 2022 • Summer Break, No Classes July 4, 2022 • Independence Day Observed, College Closed SUMMER 2022 Summer July 11, 2022 September 30, 2022 September 5, 2022 • Labor Day, College Closed FALL 2022 Fall October 3, 2022 December 23, 2022 November 24-25, 2022 • Thanksgiving, College Closed December 24, 2022-January 8, 2023 • Winter Break, No Classes December 26, 2022 • Christmas Observed, College Closed *All dates are subject to change without notice. SOUTHERNTECH.EDU 3 MODULAR TERMS* Term ID Class Start Date Class End Date Holidays / Breaks WINTER 2021 Winter A January 11, 2021 February 4, 2021 January 18, 2021 • Martin Luther King Jr. -
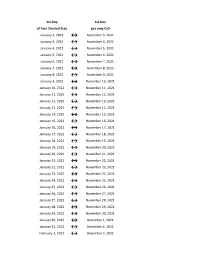
Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 2, 2022 ↔ November 3, 2021 January 3, 2022 ↔ November 4, 2021 January 4, 2022 ↔ November 5, 2021 January 5, 2022 ↔ November 6, 2021 January 6, 2022 ↔ November 7, 2021 January 7, 2022 ↔ November 8, 2021 January 8, 2022 ↔ November 9, 2021 January 9, 2022 ↔ November 10, 2021 January 10, 2022 ↔ November 11, 2021 January 11, 2022 ↔ November 12, 2021 January 12, 2022 ↔ November 13, 2021 January 13, 2022 ↔ November 14, 2021 January 14, 2022 ↔ November 15, 2021 January 15, 2022 ↔ November 16, 2021 January 16, 2022 ↔ November 17, 2021 January 17, 2022 ↔ November 18, 2021 January 18, 2022 ↔ November 19, 2021 January 19, 2022 ↔ November 20, 2021 January 20, 2022 ↔ November 21, 2021 January 21, 2022 ↔ November 22, 2021 January 22, 2022 ↔ November 23, 2021 January 23, 2022 ↔ November 24, 2021 January 24, 2022 ↔ November 25, 2021 January 25, 2022 ↔ November 26, 2021 January 26, 2022 ↔ November 27, 2021 January 27, 2022 ↔ November 28, 2021 January 28, 2022 ↔ November 29, 2021 January 29, 2022 ↔ November 30, 2021 January 30, 2022 ↔ December 1, 2021 January 31, 2022 ↔ December 2, 2021 February 1, 2022 ↔ December 3, 2021 1st Day 1st Day of Your Desired Stay you may Call February 2, 2022 ↔ December 4, 2021 February 3, 2022 ↔ December 5, 2021 February 4, 2022 ↔ December 6, 2021 February 5, 2022 ↔ December 7, 2021 February 6, 2022 ↔ December 8, 2021 February 7, 2022 ↔ December 9, 2021 February 8, 2022 ↔ December 10, 2021 February 9, 2022 ↔ December 11, 2021 February 10, 2022 ↔ December 12, 2021 February -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
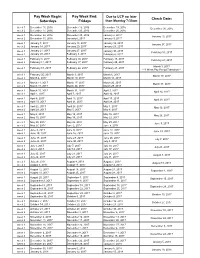
Pay Week Begin: Saturdays Pay Week End: Fridays Check Date
Pay Week Begin: Pay Week End: Due to UCP no later Check Date: Saturdays Fridays than Monday 7:30am week 1 December 10, 2016 December 16, 2016 December 19, 2016 December 30, 2016 week 2 December 17, 2016 December 23, 2016 December 26, 2016 week 1 December 24, 2016 December 30, 2016 January 2, 2017 January 13, 2017 week 2 December 31, 2016 January 6, 2017 January 9, 2017 week 1 January 7, 2017 January 13, 2017 January 16, 2017 January 27, 2017 week 2 January 14, 2017 January 20, 2017 January 23, 2017 January 21, 2017 January 27, 2017 week 1 January 30, 2017 February 10, 2017 week 2 January 28, 2017 February 3, 2017 February 6, 2017 week 1 February 4, 2017 February 10, 2017 February 13, 2017 February 24, 2017 week 2 February 11, 2017 February 17, 2017 February 20, 2017 March 3, 2017 week 1 February 18, 2017 February 24, 2017 February 27, 2017 ***1 Week Pay Period Transition*** week 1 February 25, 2017 March 3, 2017 March 6, 2017 March 17, 2017 week 2 March 4, 2017 March 10, 2017 March 13, 2017 week 1 March 11, 2017 March 17, 2017 March 20, 2017 March 31, 2017 week 2 March 18, 2017 March 24, 2017 March 27, 2017 week 1 March 25, 2017 March 31, 2017 April 3, 2017 April 14, 2017 week 2 April 1, 2017 April 7, 2017 April 10, 2017 week 1 April 8, 2017 April 14, 2017 April 17, 2017 April 28, 2017 week 2 April 15, 2017 April 21, 2017 April 24, 2017 week 1 April 22, 2017 April 28, 2017 May 1, 2017 May 12, 2017 week 2 April 29, 2017 May 5, 2017 May 8, 2017 week 1 May 6, 2017 May 12, 2017 May 15, 2017 May 26, 2017 week 2 May 13, 2017 May 19, 2017 May