(Trichoptera: Limnephilidae) Larvae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Balance of Field-Excavated Aestivating Australian Desert Frogs
3309 The Journal of Experimental Biology 209, 3309-3321 Published by The Company of Biologists 2006 doi:10.1242/jeb.02393 Water balance of field-excavated aestivating Australian desert frogs, the cocoon- forming Neobatrachus aquilonius and the non-cocooning Notaden nichollsi (Amphibia: Myobatrachidae) Victoria A. Cartledge1,*, Philip C. Withers1, Kellie A. McMaster1, Graham G. Thompson2 and S. Don Bradshaw1 1Zoology, School of Animal Biology, MO92, University of Western Australia, Crawley, Western Australia 6009, Australia and 2Centre for Ecosystem Management, Edith Cowan University, 100 Joondalup Drive, Joondalup, Western Australia 6027, Australia *Author for correspondence (e-mail: [email protected]) Accepted 19 June 2006 Summary Burrowed aestivating frogs of the cocoon-forming approaching that of the plasma. By contrast, non-cocooned species Neobatrachus aquilonius and the non-cocooning N. aquilonius from the dune swale were fully hydrated, species Notaden nichollsi were excavated in the Gibson although soil moisture levels were not as high as calculated Desert of central Australia. Their hydration state (osmotic to be necessary to maintain water balance. Both pressure of the plasma and urine) was compared to the species had similar plasma arginine vasotocin (AVT) moisture content and water potential of the surrounding concentrations ranging from 9.4 to 164·pg·ml–1, except for soil. The non-cocooning N. nichollsi was consistently found one cocooned N. aquilonius with a higher concentration of in sand dunes. While this sand had favourable water 394·pg·ml–1. For both species, AVT showed no relationship potential properties for buried frogs, the considerable with plasma osmolality over the lower range of plasma spatial and temporal variation in sand moisture meant osmolalities but was appreciably increased at the highest that frogs were not always in positive water balance with osmolality recorded. -

Species Traits Affect Phenological Responses to Climate Change in A
www.nature.com/scientificreports OPEN Species traits afect phenological responses to climate change in a butterfy community Konstantina Zografou1*, Mark T. Swartz2, George C. Adamidis1, Virginia P. Tilden2, Erika N. McKinney2 & Brent J. Sewall1 Diverse taxa have undergone phenological shifts in response to anthropogenic climate change. While such shifts generally follow predicted patterns, they are not uniform, and interspecifc variation may have important ecological consequences. We evaluated relationships among species’ phenological shifts (mean fight date, duration of fight period), ecological traits (larval trophic specialization, larval diet composition, voltinism), and population trends in a butterfy community in Pennsylvania, USA, where the summer growing season has become warmer, wetter, and longer. Data were collected over 7–19 years from 18 species or species groups, including the extremely rare eastern regal fritillary Speyeria idalia idalia. Both the direction and magnitude of phenological change over time was linked to species traits. Polyphagous species advanced and prolonged the duration of their fight period while oligophagous species delayed and shortened theirs. Herb feeders advanced their fight periods while woody feeders delayed theirs. Multivoltine species consistently prolonged fight periods in response to warmer temperatures, while univoltine species were less consistent. Butterfies that shifted to longer fight durations, and those that had polyphagous diets and multivoltine reproductive strategies tended to decline in population. Our results suggest species’ traits shape butterfy phenological responses to climate change, and are linked to important community impacts. Phenological changes are among the most noticeable responses by plants and animals to anthropogenic climate change1–3. Although some taxa may fail to respond, or respond in ways that are maladaptive4, others may undergo evolutionary change or respond via phenotypic plasticity 5. -

WAGONER, KAIRA MALINDA, M.S. Identification of Morphologic And
WAGONER, KAIRA MALINDA, M.S . Identification of Morphologic and Chemical Markers of Aestivation Conditions in Female Anopheles gambiae Mosquitoes . (2011) Directed by Dr . Tovi Lehmann and Dr . Gideon Wasserberg . 51 pp. Increased understanding of the dry season survival mechanisms of Anopheles gambiae (An. gambiae ) in semi-arid regions could benefit vector control efforts by identifying weak links in the transmission cycle of malaria . In this study we examine effect of seasonal indicators on morphologic and chemical characteristics known to contribute to suppression of water loss in mosquitoes . An. gambiae body size (indexed by wing length), mesothoracic spiracular index ((spiracle-length/wing-length)*100), and cuticular hydrocarbon (CHC) profiles were examined for their ability to differentiate mosquitoes exposed to aestivating and non-aestivating conditions in a laboratory setting . Mosquitoes exposed to aestivating conditions exhibited larger wing lengths, larger mesothoracic spiracular indices, and greater total CHC amount (standardized) than mosquitoes exposed to non-aestivating conditions . Total CHC amount increased with both mating and age . Mean n-alkane retention time (a measure of mean n-alkane chain- length) was lower in mosquitoes exposed to aestivating conditions, and increased with age. Individual CHC peaks were examined, and several CHCs were identified as potential biomarkers of aestivation, age, and insemination status . This study indicates that aestivation status of nulliparous female An. gambiae can be determined -

Hibernation Information Booklet
Hibernation Hibernation Information Booklet Hibernation Information Hibernation is a worrying time of year for most tortoise owners, so we hope that this guide will help to clear up some of the myths and answer some of the questions that you may have. If followed correctly, this guide should provide you with a successful hibernation technique and also take away the worry and stress that surrounds hibernation. Our guide will discuss what hibernation is, good health and good preparation and why it is beneficial for your tortoise. Planned preparation is absolutely fundamental to a good, successful hibernation. Unfortunately, an ill or deceased tortoise coming out of hibernation can be the result of a lack of knowledge during preparation, inadequate housing or inappropriate temperature control over the hibernating period. Hibernation is an important part of natural behaviour for tortoises in the wild so if you have a hibernating species then it is essential for good health, long life and successful breeding that you allow this natural process. What is hibernation? Tortoises are cold blooded (poikilothermic) and so in the wild, when the summer is over, and the winter months are drawing closer they begin a wind down process to cope with the drop in temperatures. The metabolic activity of a hibernating tortoise is extremely low as they ‘sleep’, during the hibernating period their brain is not active, and noise or movement will not easily wake a hibernating tortoise. Hibernating breeds of tortoises are inset with the need to hibernate. It is naturally in their biology and forms part of their essential yearly cycle. -

Mechanisms Underlying Inhibition of Muscle Disuse Atrophy During Aestivation in the Green-Striped Burrowing Frog, Cyclorana Alboguttata
Mechanisms underlying inhibition of muscle disuse atrophy during aestivation in the green-striped burrowing frog, Cyclorana alboguttata Beau Daniel Reilly Bachelor of Marine Studies (Hons.) A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2014 School of Biological Sciences Abstract In most mammals, extended inactivity or immobilisation of skeletal muscle (e.g. bed- rest, limb-casting or hindlimb unloading) results in muscle disuse atrophy, a process which is characterised by the loss of skeletal muscle mass and function. In stark contrast, animals that experience natural bouts of prolonged muscle inactivity, such as hibernating mammals and aestivating frogs, consistently exhibit limited or no change in either skeletal muscle size or contractile performance. While many of the factors regulating skeletal muscle mass are known, little information exists as to what mechanisms protect against muscle atrophy in some species. Green-striped burrowing frogs (Cyclorana alboguttata) survive in arid environments by burrowing underground and entering into a deep, prolonged metabolic depression known as aestivation. Throughout aestivation, C. alboguttata is immobilised within a cast-like cocoon of shed skin and ceases feeding and moving. Remarkably, these frogs exhibit very little muscle atrophy despite extended disuse and fasting. The overall aim of the current research study was to gain a better understanding of the physiological, cellular and molecular basis underlying resistance to muscle disuse atrophy in C. alboguttata. The first aim of this study was to develop a genomic resource for C. alboguttata by sequencing and functionally characterising its skeletal muscle transcriptome, and to conduct gene expression profiling to identify transcriptional pathways associated with metabolic depression and maintenance of muscle function in aestivating burrowing frogs. -

Winter Time in Nature
Classification Theme: To teach participants how to classify animals based on similarities they share. Background Learn the basics of the seven levels of calissifcation, Kingdom, Phylum, Class, Order Family, Genus and Species. (King Philip Come Out For Goodness Sake) Age K-5 The two mail Kingdoms are plants and animals. Four other Kingdoms include bacteria, Time 60 min archaebacterial, fungi and protozoa. Outline Phylum is separated into 30 phyla for animals. Phylum Chordata is characterized as Background the animals with backbones (Vertebrates) (chordate looks like a cord). Phylum Arthropods (Invertebrates) contains insects, spiders or any other animal with Activities: segmented bodies. They also have an exoskeleton on the outside of their bodies. Introduction Migration Class is separated into more sections within the Phylum. Example, Phylum Chordata Hibernation (animals with backbones) is then separated into birds, mammals and reptiles. Brumation Active animals Order is smaller groups within the different classes. For example, Lepidoptera is the Wrap up order of moths and butterflies. Lepidoptera is under the class insects. Carnivora is the Extra order of Mammalia. Information Family is often Speculated about as many different sources will disagree on the family of the animal. The family of dogs is Canidae (carnivorous mammals that include wolves, jackal, foxes, coyote and the domestic dog) Genus may only have one or two animals in it. If the animals are from the same genus, they are closely related. They may even look alike. When the genus is written it is capitalized and italicized. The genus of dogs and wolves is Canis. Species is when two animals can breed together successfully. -

Eco-Physiological Adaptation of the Land Snail Achatina Achatina (Gastropoda: Pulmonata) in Tropical Agro-Ecosystem
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector The Journal of Basic & Applied Zoology (2014) 67,48–57 The Egyptian German Society for Zoology The Journal of Basic & Applied Zoology www.egsz.org www.sciencedirect.com Eco-physiological adaptation of the land snail Achatina achatina (Gastropoda: Pulmonata) in tropical agro-ecosystem Christian O. Chukwuka *, Vincent C. Ejere, Chinweike N. Asogwa, Emmanuel I. Nnamonu, Ogochukwu C. Okeke, Elijah I. Odii, Godwin C. Ugwu, Loretta C. Okanya, Chidinma A. Levi Department of Zoology and Environmental Biology, University of Nigeria, Nsukka, Nigeria Received 24 January 2014; revised 28 May 2014; accepted 13 June 2014 Available online 27 October 2014 KEYWORDS Abstract The survival of land snails in an adverse environmental condition depends on the integral Eco-physiology; physiological, morphological and behavioural adaptations. These adaptations are essential in Adaptation; understanding the species-specific habitat requirements and in predicting their environmental Agro-ecosystem; responses. In this study, the monthly and the periodic patterns of eco-physiological adaptation Tropics; of land snail, Achatina achatina in Nsukka tropical agro-ecosystem were assessed from December Gastropods; 2012 to July 2013. Standard methods were employed in sampling the land snail and determination Aestivation of the water content, biochemical fuel reserves and enzyme concentrations of the samples. The pres- ent results showed that lipids were high at the beginning of aestivation and depleted as the aestiva- tion progressed. Glycogen was significantly low throughout the aestivation months (December– March) and increased in the active months (April–July). Protein content recorded a definite pattern all through the months studied. -

Diapause/ Dormancy
Chapter 6 Diapause/ Dormancy Ivo Hodek Institute of Entomology, Academy of Sciences, CZ 370 05, Cˇ esk é Bud eˇ jovice, Czech Republic Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), First Edition. Edited by I. Hodek, H.F. van Emden, A. Honeˇk. © 2012 Blackwell Publishing Ltd. Published 2012 by Blackwell Publishing Ltd. 275 6.1 INTRODUCTION: MECHANISMS induces 50% response, i.e. in half of the sample AND DEFINITIONS diapause is induced, in the other half it is not. It should be stressed that potentially multivoltine species Some generalities need to be explained to make the may show a univoltine life cycle in regions where the reading of this chapter easier. The adaptive functions period of suitable conditions is unfavourably short. By of diapause are: (i) to synchronize the development contrast, ‘ obligatory ’ diapause is entered by virtually of active stages with favourable conditions and (ii) to every individual in each generation of the so - called enhance the survival potential during unfavourable obligatory univoltines, regardless of the environ- periods. The modern defi nitions were coined by Lees ment (Lees 1955 ). (1955) . He defi ned quiescence as direct inhibition of The original defi nition of obligatory diapause has development, caused by the direct effect of ambient become outdated by experiments with changing conditions (low temperature, lack of humidity), which photoperiods. Changing conditions (e.g. exposure to can be terminated immediately by favourable condi- short days followed by long days) for insects entering tions. Diapause is caused by conditions which do not ‘ obligatory ’ diapause under, for example, a constant directly prevent development, but which are merely photoperiod in the laboratory, may be a prerequisite for signals of seasonal changes (cues, seasonal tokens; a their development. -

Facultative Aestivation in a Tropical Freshwater Turtle Chelodina Rugosa
FACULTATIVE AESTIVATION IN A TROPICAL FRESHWATER TURTLE CHELODINA RUGOSA G. C. GRIGG,* K. JOHANSEN,† P. HARLOW,* L. A. BEARD* and L. E. TAPLIN‡ *Zoology A.08, The University of Sydney, NSW 2006, Australia. Telephone: 692-2222, †Department of Zoophysiology, University of Aarhus, Aarhus, DK 8000, Denmark and ‡Queensland National Parks and Wildlife Service, Pallarenda, Queensland 4810, Australia Abstract-1. Chelodina rugosa dug from aestivation sites at the end of the dry season were immediately alert and well coordinated. 2. Compared with non-aestivating animals, aestivating turtles had 20% higher plasma osmotic pressure and 7% higher sodium. Coupled with a small, but significant weight gain upon return to the water, this suggested the occurrence of minor dehydration in aestivating animals. 3. Plasma lactate levels of aestivating animals were low, averaging 1.99 mmol/1, consistent with aerobic rather than anaerobic metabolism having sustained their long period under ground. 4. No evidence was seen of dramatic physiological specialization. Aestivation in this species is interpreted as a primarily behavioural adaptation, made possible by typically reptilian abilities to tolerate a wide range in plasma electrolytes and to survive long periods without feeding. INTRODUCTION The northern snake-necked tortoise, Chelodina rugosa (Chelidae), occurs in tropical Australia from Cape York to the Kimberley district of western Australia, where it is found in freshwater swamps, billabongs, waterholes and slow-flowing rivers (Cogger, 1975). The climate throughout its range is markedly seasonal, with the summer monsoon lasting from about November to March and little or no rain in the intervening dry season. The summers are hot and humid, winters warm and less humid. -
Hibernation: Endotherms Secondary Article
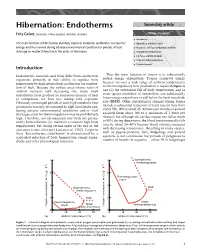
Hibernation: Endotherms Secondary article Fritz Geiser, University of New England, Armidale, Australia Article Contents . Introduction The main function of hibernation and daily torpor in mammals and birds is to conserve . Hibernation and Daily Torpor energy and thus survive during adverse environmental conditions or periods of food . Occurrence of Torpor in Mammals and Birds shortage no matter if they live in the arctic or the tropics. Preparation for Hibernation . Fat Stores and Dietary Lipids . Body Size and its Implications . Periodic Arousals Introduction Endothermic mammals and birds differ from ectothermic Thus the main function of torpor is to substantially organisms primarily in their ability to regulate body reduce energy expenditure. Torpor conserves energy temperature by high internal heat production via combus- because: (i) over a wide range of ambient temperatures tion of fuels. Because the surface area/volume ratio of no thermoregulatory heat production is required (Figure 1) animals increases with decreasing size, many small and (ii) the substantial fall of body temperature, and in endotherms must produce an enormous amount of heat some species inhibition of metabolism, can substantially to compensate for heat loss during cold exposure. lower energy expenditure to well below the basal metabolic Obviously, prolonged periods of such high metabolic heat rate (BMR). Other physiological changes during torpor production can only be sustained by high food intake and, include a substantial reduction of heart rate (in bats from during adverse environmental conditions and/or food about 500–900 to about 20–40 beats per minute; in ground shortages, costs for thermoregulation may be prohibitively squirrels from about 300 to a minimum of 3 beats per high. -

Field Guide for the Biological Control of Weeds in Eastern North America
US Department TECHNOLOGY of Agriculture TRANSFER FIELD GUIDE FOR THE BIOLOGICAL CONTROL OF WEEDS IN EASTERN NORTH AMERICA Rachel L. Winston, Carol B. Randall, Bernd Blossey, Philip W. Tipping, Ellen C. Lake, and Judy Hough-Goldstein Forest Health Technology FHTET-2016-04 Enterprise Team April 2017 The Forest Health Technology Enterprise Team (FHTET) was created in 1995 by the Deputy Chief for State and Private Forestry, USDA, Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ Cover photos: Purple loosestrife (Jennifer Andreas, Washington State University Extension), Galerucella calmariensis (David Cappaert, Michigan State University, bugwood.org), tropical soda apple ((J. Jeffrey Mullahey, University of Florida, bugwood.org), Gratiana boliviana (Rodrigo Diaz, Louisiana State University), waterhyacinth (Chris Evans, University of Illinois, bugwood.org), Megamelus scutellaris (Jason D. Stanley, USDA ARS, bugwood.org), mile-a-minute weed (Leslie J. Mehrhoff, University of Connecticut, bugwood.org), Rhinoncomimus latipes (Amy Diercks, bugwood.org) How to cite this publication: Winston, R.L., C.B. Randall, B. Blossey, P.W. Tipping, E.C. Lake, and J. Hough-Goldstein. 2017. Field Guide for the Biological Control of Weeds in Eastern North America. USDA Forest Service, Forest Health Technology Enterprise Team, Morgantown, West Virginia. FHTET-2016-04. In accordance with -

Thermal Variation, Thermal Extremes and the Physiological Performance of Individuals W
© 2015. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2015) 218, 1956-1967 doi:10.1242/jeb.114926 REVIEW Thermal variation, thermal extremes and the physiological performance of individuals W. Wesley Dowd1,*, Felicia A. King2 and Mark W. Denny2 ABSTRACT and biochemists have focused on the mean of performance, often In this review we consider how small-scale temporal and spatial glossing over variation (Bennett, 1987). However, this focus draws variation in body temperature, and biochemical/physiological attention away from the fact that contemporary patterns in variation among individuals, affect the prediction of organisms’ biochemistry and physiology are products of selection on the performance in nature. For ‘normal’ body temperatures – benign variation that existed in the ancestors of present-day organisms. temperatures near the species’ mean – thermal biology traditionally When confronted with changes in their environment, different uses performance curves to describe how physiological capabilities individuals within a species can be quite variable in their response vary with temperature. However, these curves, which are typically (Crawford and Oleksiak, 2007; Krebs and Feder, 1997; Nikinmaa and measured under static laboratory conditions, can yield incomplete or Waser, 2007; Williams, 2008). The sources and consequences of this ’ inaccurate predictions of how organisms respond to natural patterns variation are pivotal to understanding species potentials to cope with of temperature variation. For example, scale transition theory predicts changing environments (Whitehead and Crawford, 2006). As Steven that, in a variable environment, peak average performance is lower Jay Gould (1985) eloquently put it: and occurs at a lower mean temperature than the peak of statically measured performance.