Fluoride Geochemistry of Groundwater in Nalhati-1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

W.B.C.S.(Exe.) Officers of West Bengal Cadre
W.B.C.S.(EXE.) OFFICERS OF WEST BENGAL CADRE Sl Name/Idcode Batch Present Posting Posting Address Mobile/Email No. 1 ARUN KUMAR 1985 COMPULSORY WAITING NABANNA ,SARAT CHATTERJEE 9432877230 SINGH PERSONNEL AND ROAD ,SHIBPUR, (CS1985028 ) ADMINISTRATIVE REFORMS & HOWRAH-711102 Dob- 14-01-1962 E-GOVERNANCE DEPTT. 2 SUVENDU GHOSH 1990 ADDITIONAL DIRECTOR B 18/204, A-B CONNECTOR, +918902267252 (CS1990027 ) B.R.A.I.P.R.D. (TRAINING) KALYANI ,NADIA, WEST suvendughoshsiprd Dob- 21-06-1960 BENGAL 741251 ,PHONE:033 2582 @gmail.com 8161 3 NAMITA ROY 1990 JT. SECY & EX. OFFICIO NABANNA ,14TH FLOOR, 325, +919433746563 MALLICK DIRECTOR SARAT CHATTERJEE (CS1990036 ) INFORMATION & CULTURAL ROAD,HOWRAH-711102 Dob- 28-09-1961 AFFAIRS DEPTT. ,PHONE:2214- 5555,2214-3101 4 MD. ABDUL GANI 1991 SPECIAL SECRETARY MAYUKH BHAVAN, 4TH FLOOR, +919836041082 (CS1991051 ) SUNDARBAN AFFAIRS DEPTT. BIDHANNAGAR, mdabdulgani61@gm Dob- 08-02-1961 KOLKATA-700091 ,PHONE: ail.com 033-2337-3544 5 PARTHA SARATHI 1991 ASSISTANT COMMISSIONER COURT BUILDING, MATHER 9434212636 BANERJEE BURDWAN DIVISION DHAR, GHATAKPARA, (CS1991054 ) CHINSURAH TALUK, HOOGHLY, Dob- 12-01-1964 ,WEST BENGAL 712101 ,PHONE: 033 2680 2170 6 ABHIJIT 1991 EXECUTIVE DIRECTOR SHILPA BHAWAN,28,3, PODDAR 9874047447 MUKHOPADHYAY WBSIDC COURT, TIRETTI, KOLKATA, ontaranga.abhijit@g (CS1991058 ) WEST BENGAL 700012 mail.com Dob- 24-12-1963 7 SUJAY SARKAR 1991 DIRECTOR (HR) BIDYUT UNNAYAN BHAVAN 9434961715 (CS1991059 ) WBSEDCL ,3/C BLOCK -LA SECTOR III sujay_piyal@rediff Dob- 22-12-1968 ,SALT LAKE CITY KOL-98, PH- mail.com 23591917 8 LALITA 1991 SECRETARY KHADYA BHAWAN COMPLEX 9433273656 AGARWALA WEST BENGAL INFORMATION ,11A, MIRZA GHALIB ST. agarwalalalita@gma (CS1991060 ) COMMISSION JANBAZAR, TALTALA, il.com Dob- 10-10-1967 KOLKATA-700135 9 MD. -

Date Wise Details of Covid Vaccination Session Plan
Date wise details of Covid Vaccination session plan Name of the District: Darjeeling Dr Sanyukta Liu Name & Mobile no of the District Nodal Officer: Contact No of District Control Room: 8250237835 7001866136 Sl. Mobile No of CVC Adress of CVC site(name of hospital/ Type of vaccine to be used( Name of CVC Site Name of CVC Manager Remarks No Manager health centre, block/ ward/ village etc) Covishield/ Covaxine) 1 Darjeeling DH 1 Dr. Kumar Sariswal 9851937730 Darjeeling DH COVAXIN 2 Darjeeling DH 2 Dr. Kumar Sariswal 9851937730 Darjeeling DH COVISHIELD 3 Darjeeling UPCH Ghoom Dr. Kumar Sariswal 9851937730 Darjeeling UPCH Ghoom COVISHIELD 4 Kurseong SDH 1 Bijay Sinchury 7063071718 Kurseong SDH COVAXIN 5 Kurseong SDH 2 Bijay Sinchury 7063071718 Kurseong SDH COVISHIELD 6 Siliguri DH1 Koushik Roy 9851235672 Siliguri DH COVAXIN 7 SiliguriDH 2 Koushik Roy 9851235672 SiliguriDH COVISHIELD 8 NBMCH 1 (PSM) Goutam Das 9679230501 NBMCH COVAXIN 9 NBCMCH 2 Goutam Das 9679230501 NBCMCH COVISHIELD 10 Matigara BPHC 1 DR. Sohom Sen 9435389025 Matigara BPHC COVAXIN 11 Matigara BPHC 2 DR. Sohom Sen 9435389025 Matigara BPHC COVISHIELD 12 Kharibari RH 1 Dr. Alam 9804370580 Kharibari RH COVAXIN 13 Kharibari RH 2 Dr. Alam 9804370580 Kharibari RH COVISHIELD 14 Naxalbari RH 1 Dr.Kuntal Ghosh 9832159414 Naxalbari RH COVAXIN 15 Naxalbari RH 2 Dr.Kuntal Ghosh 9832159414 Naxalbari RH COVISHIELD 16 Phansidewa RH 1 Dr. Arunabha Das 7908844346 Phansidewa RH COVAXIN 17 Phansidewa RH 2 Dr. Arunabha Das 7908844346 Phansidewa RH COVISHIELD 18 Matri Sadan Dr. Sanjib Majumder 9434328017 Matri Sadan COVISHIELD 19 SMC UPHC7 1 Dr. Sanjib Majumder 9434328017 SMC UPHC7 COVAXIN 20 SMC UPHC7 2 Dr. -

1543927662BAY Writte
_, tJutba ~arbbaman .liUa JJari~bab Court Compound, Bardhaman-713101 zp [email protected] Tel: 0342-2662400 Fax-0342-2663327 Memo No :- 2() 9 a IPBZP Dated, 04/l2./2018 From :- Deputy Secretary, Purba Bardhaman Zilla Parishad To: District Information Officer, Purba Bardhaman Sir, Enclosed please find herewith the list of candidates eligible to appear in the written examination for the recruitment to post of District Coordinator & Technical Assistant on the is" December, 2018 from 10:00 AM. You are requested to upload the same official website of Purba Bardhaman. Deputy Secretary, Purba Bardhaman Zilla Parishad MemoNo :- QS.,o !3/PBZP Dated, 4 I J 2./2018 Copy forwarded for information and necessaryaction to :- I) DIA, Purba Bardhaman Zilla Parishad for wide circulation through Zilla Parishadwebsite II) CA to District Magistrate, Purba Bardhaman for kind perusal of the DM. Purba Bardhaman. III) CA to Additional Executive officer, Purba Bardhaman Zilla Parishad for kind perusal of the AEO. Purba Bardhaman Zilla Parishad . Deputy Secretary, Purba Bardhaman Zilla Parishad E:\.6.rjun important files\IAY-communication-17-18_arjun updated.docx Father 51 Apply for Name Name/Husband/Guard ViII / City PO P5 District PIN No the Post ian's 85-Balidanga, District Co- Purba 1 Arnab Konar Prasanta kr. Konar Nazrulpally Sripally Burdwan Sadar 713103 ordinator Bardhaman Boronipur District Co- Purba 2 Partha Kumar Gour Chandra Kumar Jyotchilam Bolpur Raina 713103 ordinator Bardhaman District Co- Purba 3 Sraboni Pal Mondal Mahadeb Mondal Askaran Galsi Galsi 713406 ordlnator Bardhaman District Co- Patuli Station Purba 4 Dhrubajyoti Shil Sunil Kumar Shil Patuli Station Bazar Purbasthali 713512 ordinator Bazar Bardhaman District Co- Lakshmi Narayan Paschim 5 Antu 5arkar Khandra Khandra Andal 713363 ordinator Sarkar Bardhaman District Co- Purba 6 Sk Amiruddin Sk Johiruddin East Bardhaman Bardhaman Bardhaman 713101 ordinator Bardhaman District Co- Purba 7 Sujit Malik Lt. -

Human Resource Development of Birbhum District – a Critical Study
IOSR Journal Of Humanities And Social Science (IOSR-JHSS) Volume 19, Issue 2, Ver. V (Feb. 2014), PP 62-67 e-ISSN: 2279-0837, p-ISSN: 2279-0845. www.iosrjournals.org Human Resource Development of Birbhum District – A Critical Study 1Debasish Roy, 2Anushri Mondal M.Phil Scholar in Rabindra Bharati University, CSIR NET in Earth, Atmospheric, Ocean and Planetary Science. UGC NET in Geography, Rajiv Gandhi National Junior Research Fellow and Asst. Teacher Ahiran Hemangini Vidyayatan High school., M.A, NET Abstract: In this paper we discuss the human resource development of Birbhum District. The data have been collected from District Statistical Handbook, District census report of 2001 and District Human Development Report 2009.A large part of the Birbhum District is still backward with respect to human resource development. Aim of this paper is to study the cause of the backwardness of this district. “HRD is the process of determining the optimum methods of developing and improving the human resources of an organization and the systematic improvement of the performance of employees through training, education and development and leadership for the mutual attainment of organizational and personal goals” (Smith). HRD is an important topic of present time. It is considered by management professionals, as sub discipline of Human Resource Management( HRM), but many researchers have, broadened the scope and integrated the concept of HRD by looking it from socioeconomic angle and giving it other dimension such as physical, intellectual, psychological, social, political, moral and spiritual development. I. Introduction: Human Resource Development is the ultimate goal of National Development. HRD is the process of increasing the knowledge, the skills, and the capacities of all the people in a society. -

List of Gram Panchayat Under Social Sector Ii of Local Audit Department
LIST OF GRAM PANCHAYAT UNDER SOCIAL SECTOR II OF LOCAL AUDIT DEPARTMENT Last SL. Audit DISTRICT BLOCK GP NO ed up to 2015- 1 ALIPURDUAR ALIPURDUAR-I BANCHUKAMARI 16 2015- 2 ALIPURDUAR ALIPURDUAR-I CHAKOWAKHETI 16 2015- 3 ALIPURDUAR ALIPURDUAR-I MATHURA 16 2015- 4 ALIPURDUAR ALIPURDUAR-I PARORPAR 16 2015- 5 ALIPURDUAR ALIPURDUAR-I PATLAKHAWA 16 2015- 6 ALIPURDUAR ALIPURDUAR-I PURBA KANTHALBARI 16 2015- 7 ALIPURDUAR ALIPURDUAR-I SHALKUMAR-I 16 2015- 8 ALIPURDUAR ALIPURDUAR-I SHALKUMAR-II 16 2015- 9 ALIPURDUAR ALIPURDUAR-I TAPSIKHATA 16 2015- 10 ALIPURDUAR ALIPURDUAR-I VIVEKANDA-I 16 2015- 11 ALIPURDUAR ALIPURDUAR-I VIVEKANDA-II 16 2015- 12 ALIPURDUAR ALIPURDUAR-II BHATIBARI 16 2015- 13 ALIPURDUAR ALIPURDUAR-II CHAPORER PAR-I 16 2015- 14 ALIPURDUAR ALIPURDUAR-II CHAPORER PAR-II 16 2015- 15 ALIPURDUAR ALIPURDUAR-II KOHINOOR 16 2015- 16 ALIPURDUAR ALIPURDUAR-II MAHAKALGURI 16 2015- 17 ALIPURDUAR ALIPURDUAR-II MAJHERDABRI 16 2015- 18 ALIPURDUAR ALIPURDUAR-II PAROKATA 16 2015- 19 ALIPURDUAR ALIPURDUAR-II SHAMUKTALA 16 2015- 20 ALIPURDUAR ALIPURDUAR-II TATPARA-I 16 2015- 21 ALIPURDUAR ALIPURDUAR-II TATPARA-II 16 2015- 22 ALIPURDUAR ALIPURDUAR-II TURTURI 16 2015- 23 ALIPURDUAR FALAKATA DALGAON 16 2016- 24 ALIPURDUAR FALAKATA DEOGAON 18 2015- 25 ALIPURDUAR FALAKATA DHANIRAMPUR-I 16 2015- 26 ALIPURDUAR FALAKATA DHANIRAMPUR-II 16 2015- 27 ALIPURDUAR FALAKATA FALAKATA-I 16 2015- 28 ALIPURDUAR FALAKATA FALAKATA-II 16 2016- 29 ALIPURDUAR FALAKATA GUABARNAGAR 18 2015- 30 ALIPURDUAR FALAKATA JATESWAR-I 16 2015- 31 ALIPURDUAR FALAKATA JATESWAR-II 16 2016- -

Name of DDO/Hoo ADDRESS-1 ADDRESS CITY PIN SECTION REF
Name of DDO/HoO ADDRESS-1 ADDRESS CITY PIN SECTION REF. NO. BARCODE DATE THE SUPDT OF POLICE (ADMIN),SPL INTELLIGENCE COUNTER INSURGENCY FORCE ,W B,307,GARIA GROUP MAIN ROAD KOLKATA 700084 FUND IX/OUT/33 ew484941046in 12-11-2020 1 BENGAL GIRL'S BN- NCC 149 BLCK G NEW ALIPUR KOLKATA 0 0 KOLKATA 700053 FD XIV/D-325 ew460012316in 04-12-2020 2N BENAL. GIRLS BN. NCC 149, BLOCKG NEW ALIPORE KOL-53 0 NEW ALIPUR 700053 FD XIV/D-267 ew003044527in 27-11-2020 4 BENGAL TECH AIR SAQ NCC JADAVPUR LIMIVERSITY CAMPUS KOLKATA 0 0 KOLKATA 700032 FD XIV/D-313 ew460011823in 04-12-2020 4 BENGAL TECH.,AIR SQN.NCC JADAVPUR UNIVERSITY CAMPUS, KOLKATA 700036 FUND-VII/2019-20/OUT/468 EW460018693IN 26-11-2020 6 BENGAL BATTALION NCC DUTTAPARA ROAD 0 0 N.24 PGS 743235 FD XIV/D-249 ew020929090in 27-11-2020 A.C.J.M. KALYANI NADIA 0 NADIA 741235 FD XII/D-204 EW020931725IN 17-12-2020 A.O & D.D.O, DIR.OF MINES & MINERAL 4, CAMAC STREET,2ND FL., KOLKATA 700016 FUND-XIV/JAL/19-20/OUT/30 ew484927906in 14-10-2020 A.O & D.D.O, O/O THE DIST.CONTROLLER (F&S) KARNAJORA, RAIGANJ U/DINAJPUR 733130 FUDN-VII/19-20/OUT/649 EW020926425IN 23-12-2020 A.O & DDU. DIR.OF MINES & MINERALS, 4 CAMAC STREET,2ND FL., KOLKATA 700016 FUND-IV/2019-20/OUT/107 EW484937157IN 02-11-2020 STATISTICS, JT.ADMN.BULDS.,BLOCK-HC-7,SECTOR- A.O & E.O DY.SECY.,DEPTT.OF PLANNING & III, KOLKATA 700106 FUND-VII/2019-20/OUT/470 EW460018716IN 26-11-2020 A.O & EX-OFFICIO DY.SECY., P.W DEPTT. -

Nalhati Assembly West Bengal Factbook
Editor & Director Dr. R.K. Thukral Research Editor Dr. Shafeeq Rahman Compiled, Researched and Published by Datanet India Pvt. Ltd. D-100, 1st Floor, Okhla Industrial Area, Phase-I, New Delhi- 110020. Ph.: 91-11- 43580781, 26810964-65-66 Email : [email protected] Website : www.electionsinindia.com Online Book Store : www.datanetindia-ebooks.com Report No. : AFB/WB-293-0619 ISBN : 978-93-5293-755-4 First Edition : January, 2018 Third Updated Edition : June, 2019 Price : Rs. 11500/- US$ 310 © Datanet India Pvt. Ltd. All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical photocopying, photographing, scanning, recording or otherwise without the prior written permission of the publisher. Please refer to Disclaimer at page no. 164 for the use of this publication. Printed in India No. Particulars Page No. Introduction 1 Assembly Constituency at a Glance | Features of Assembly as per 1-2 Delimitation Commission of India (2008) Location and Political Maps 2 Location Map | Boundaries of Assembly Constituency in District | Boundaries 3-9 of Assembly Constituency under Parliamentary Constituency | Town & Village-wise Winner Parties- 2019, 2016, 2014, 2011 and 2009 Administrative Setup 3 District | Sub-district | Towns | Villages | Inhabited Villages | Uninhabited 10-16 Villages | Village Panchayat | Intermediate Panchayat Demographics 4 Population | Households | Rural/Urban Population | Towns and Villages by 17-18 Population Size | Sex Ratio (Total -

Urban Expansion and Population Growth of Bolpur Town in Birbhum District, West Bengal
www.ijcrt.org © 2020 IJCRT | Volume 8, Issue 5 May 2020 | ISSN: 2320-2882 URBAN EXPANSION AND POPULATION GROWTH OF BOLPUR TOWN IN BIRBHUM DISTRICT, WEST BENGAL Dr. Mahuya Sen Assistant Professor Department of Geography Birbhum Mahavidyalaya, Suri, Birbhum District,West Bengal, India Abstract: Urban growth is considered as one of the essential indicators of economic growth and development of a country. Along with the increase of population, towns have been growing rapidly in physical dimension in the past few decades. Bolpur town is one of the fast growing towns of the Birbhum District of West Bengal. Bolpur town is situated almost in the middle of the Bolpur- Sriniketan C.D. Block, which is the southernmost Block of Birbhum District. It includes world famous Santiniketan , place of Rabindranath Tagore. The objective is to study the pattern of urban expansion and rapid population growth of Bolpur town and analyze the factors behind this urbanization process. The study is based on secondary data. The relevant maps are drawn by using GIS application. Bolpur town has been grown up from a village to a municipal town. Bolpur is the only municipality in Bolpur sub-division. The decadal growth rate in 1991-2001 was 24.1%. Various economic and cultural factors are responsible for the rapid urban expansion. But variation of population growth is also found among the different wards of Bolpur Town. Index Terms - Urban expansion, Development, Population growth, Town, C.D. Block, Economy. I. INTRODUCTION India is gradually making the shift from ‘rural’ to ‘urban’, at a much slower pace than other developed nations, as the urbanisation levels of India has ‘low base’. -
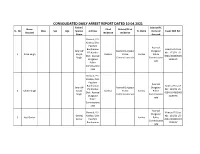
CONSOLIDATED DAILY ARREST REPORT DATED 10.04.2021 Father/ District/PC Name PS of District/PC of SL
CONSOLIDATED DAILY ARREST REPORT DATED 10.04.2021 Father/ District/PC Name PS of District/PC of SL. No Alias Sex Age Spouse Address Ps Name Name of Case/ GDE Ref. Accused residence residence Name Accused Bonkati, P.S- Kanksa, Dist- Paschim Asansol Bardhaman Kanksa PS Case Sanjit @ Asansol Durgapur Durgapur PS: Kanksa No : 123/21 US- 1 Kiran Singh Sanjib Kanksa Police Kanksa Police Dist.: Asansol 498A/304B/506/ Singh Commissionerate Commissione Durgapur 120B IPC rate Police Commissione rate Bonkati, P.S- Kanksa, Dist- Paschim Asansol Bardhaman Kanksa PS Case Sanjit @ Asansol Durgapur Durgapur PS: Kanksa No : 123/21 US- 2 Sabitri Singh Sanjib Kanksa Police Kanksa Police Dist.: Asansol 498A/304B/506/ Singh Commissionerate Commissione Durgapur 120B IPC rate Police Commissione rate Asansol Bonkati, P.S- Kanksa PS Case Durgapur Debraj Kanksa, Dist- No : 123/21 US- 3 Jyoti Sarkar Kanksa Police Sarkar Paschim 498A/304B/506/ Commissione Bardhaman 120B IPC rate Kanchanpur, P.S- Kanksa, Dist- Paschim Asansol Kanksa PS Case Lt. Bardhaman Asansol Durgapur Durgapur No : 125/21 US- 4 Gopijiban Pan Khelaram Dist.: Asansol Police Kanksa Police 46 (A) Bengal Pan Durgapur Commissionerate Commissione Excise Act, 1909 Police rate Commissione rate Asansol Raniganj PS Case Durgapur Lt.akul No : 185/21 US- 5 Uttam Gope Panchu M Raniganj Police Gope 46A Bengal Commissione Excise Act, 1909 rate Asansol Durgapur Lakhindar Lt.sakal Chittaranja Chittaranjan PS 6 M 26 Police Hembram Hembram n GDE No. 359 Commissione rate Borira PS: Kulti Dist.: Asansol Lt. Asansol Asansol Durgapur Durgapur Kulti PS Outpost 7 Bhola Gorai M 22 Haradhan Durgapur Kulti Police Kulti Police Chowrangi OP Gorai Police Commissionerate Commissione GDE No. -

District Birbhum Hydrogeological
87°15'0"E 87°30'0"E 87°45'0"E 88°0'0"E DISTRICT BIRBHUM 24°30'0"N HYDROGEOLOGICAL MAP 24°30'0"N Murarai-I Murarai-II ± Nalhati-I Nalhati-II i i Nad hman 24°15'0"N Bra 24°15'0"N Rampurhat-I Rampurhat-II d an kh ar Jh Mayureswar-I Mohammad Bazar 24°0'0"N 24°0'0"N Rajnagar Mayureswar-II M Murshidabad ayu raks hi N District adi Suri-I Sainthia Suri-II Labpur Khoyrasol Dubrajpur 23°45'0"N Legend 23°45'0"N Rock Type Alternating layers of sand,silt and clay Illambazar Bolpur Sriniketan Nanoor Hard clays impregnated with caliche nodules Laterite and lateritic soils Aj ay R iver Rajmahal trap-basalt Barddhaman District Sandstone and shale 23°30'0"N 23°30'0"N Black and grey shale with ironstone sandstone River 0 2.5 5 10 15 20 Pegmatite (unclassified) Kilometers Granite gneiss with enclaves of melamorphites District Boundary Quartzite Block Boundary Projection & Geodetic Reference System: GCS,WGS 1984 Symbol Rock Type Age Lithology Aquifer Description Hydrogeology Sand,silt and clay of flood plain, Multiple aquifer systems occur, Low to heavy-duty tubewells are Hard clays Jurassic to Late mature deltaic plain and in general, in the depth of feasible.The yield prospect is 23°15'0"N with caliche Holocene 23°15'0"N para-deltaic fan surface and 12-396m b.g.l. 7.2-250 cum/hr nodules, sand,silt, lateritic surface rajmahal trap basalt,laterite Ground water occurs,in the Dugwells and borewells or Shale,sand stone, Archaean to Soft to medium hard weathered residuum within dug-cum-borewells are feasible in silt stone, Triassic sedimentary rocks 6-12m b.g.l. -

SAR 85 Minority Affairs 2017
RegisteredPART I] No. WB/SC-247 THE KOLKATA GAZETTE, EXTRAORDINARY, AUGUST 12,No. 2017 WB(Part-I)/2017/SAR-507 1 The Kolkata Gazette Extraordinary Published by Authority ASADHA 23] TUESDAY, AUGUST 15, 2017 [SAKA 1939 PART I—Orders and Notifications by the Governor of West Bengal, the High Court, Government Treasury, etc. WEST BENGAL STATE ELECTION COMMISSION 18, Sarojini Naidu Sarani (Rawdon Street) Kolkata-700 017 No. 1063-SEC/1E-85/2017 Dated the, 15th August, 2017 NOTIFICATION WHEREAS, Municipal General Elections, 2017 to Durgapur Municipal Corporation / Dhupguri / Buniadpur / Haldia / Panskura / Nalhati Municipality / Coopers’ Camp Notified Area and Municipal Bye Elections, 2017 to Champdani Municipality (Ward No. 12) / Jhargram Municipality (Ward No. 7) were held on 13.08.2017 covering 794 Polling stations; And WHEREAS, the Commission has instructed respective DM & DMEOs and MROs for looking into every complaint received by this Commission’s office as well as at local level along with video viewing of CCTV/ Videography at the polling stations; And WHEREAS, the Commission has also instructed for detailed video viewing by respective DMEOs & MROs of the polling stations where voter turnout is more than 90% (which is 141 in number); And WHEREAS, the DMEOs of Jalpaiguri / Dakshin Dinajpur / Purba Medinipur / Nadia / Birbhum / Jhargram and Hooghly and MROs of Dhupguri / Buniadpur / Haldia / Nalhati Municipality / Coopers’ Camp Notified Area / Champdani Municipality (Ward No. 12) / Jhargram Municipality (Ward No. 7), have not recommended any re-poll in any polling station after post poll scrutiny, detailed video viewing and enquiry in Dhupguri / Buniadpur / Haldia / Nalhati Municipality / Coopers’ Camp Notified Area / Champdani Municipality (Ward No. -

From : Commissioner, Health and Family Welfare Department & Addl
GOVERNMENT OF WEST BENGAL HEALTH & FAMILY WELFARE DEPARTMENT NATIONAL HEALTH MISSION (NHM) GN -29, 1ST FLOOR, GRANTHAGAR BHAWAN, SWASTHYA BHAWAN PREMISES, SECTOR-V SALT LAKE, BIDHANNAGAR, KOLKATA - 700 091. ,~ iA 033 - 2353 - 0432, 033 - 2357 - 7930, Email ID: [email protected]; website: www.wbhealth.gov.in Memo No. H/NUHM-697/2015/4933 Date: 20.03.2017 From : Commissioner, Health and Family Welfare Department & Addl. Mission Director, NHM Government of West Bengal To Chief Medical Officer of Health (all districts) Madam / Sir, An engagement order was issued vide Order No. SHFWS/ESTD-866/2015j7831, dated: 04.01.2017 for 272 Pharmacists under NUHM. The last date of joining was 19.01.2017. Out of 272 who were given engagement, 238 have joined. The details of Pharmacist under NUHM who have reportedly joined have been enclosed (Soft copy). You are requested to confirm the list within 31st March, 2017 for filling up of vacant posts. End: As stated Yours faithfully, ~ Commissioner, H&FW & Addl. Mission Director, NHM Memo No. H/NUHM-697/2015/4933 Date: 20.03.2017 Copy forwarded for information & necessary action to : 1. IT Cell for Web posting. 2. Guard file ~ Commissioner, H&FW & Addl. Mission Director, NHM Format for submission of in position and vacancy status of Pharmacist under NUHM Recruitment Notice No: SHFWS/2015/68, dated: 08/10/2015 Date of Placeof Posting Date of Presently If resigned, 51. FullAddresswith Pin Category x Joining at District Name of the Employee DOB w (Nameof the U-PHC Joining at working date of Contact No. Remarks No.