The Deleterious Impact of Interleukin 9 to Hepatorenal Physiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Vδ2 T Cells Are a Major Source of Interleukin-9
Human Vδ2 T cells are a major source of interleukin-9 Christian Petersa, Robert Häslerb, Daniela Wescha, and Dieter Kabelitza,1 aInstitute of Immunology, University Hospital Schleswig-Holstein Campus Kiel, D-24105 Kiel, Germany; and bInstitute of Clinical Molecular Biology, University Hospital Schleswig-Holstein Campus Kiel, D-24105 Kiel, Germany Edited by Willi K. Born, National Jewish Health, Denver, CO, and accepted by Editorial Board Member Philippa Marrack September 12, 2016 (received for review May 13, 2016) Vδ2Vγ9 T cells are the dominant γδ T-cell subset in human periph- Vδ2 T cells produce different cytokines, can be converted into + eral blood. Vδ2 T cells recognize pyrophosphate molecules derived forkhead box protein P3 (FoxP3) regulatory cells (20, 21), and from microbes or tumor cells; hence, they play a role in antimicro- may acquire professional antigen-presenting capacity (22). Depend- bial and antitumor immunity. TGF-β, together with IL-15, induces ing on priming conditions, Vδ2 T cells can secrete IFN-γ,IL-4,IL- a regulatory phenotype in Vδ2 T cells, characterized by forkhead 17, and IL-22, and thus recapitulate cytokine patterns of well-defined box protein P3 (FoxP3) expression and suppressive activity on CD4 functional Th subsets (23, 24). T-cell activation. We performed a genome-wide transcriptome Here, we identified peripheral blood Vδ2Tcellsasamajorsource analysis and found that the same conditions (TGF-β plus IL-15) of IL-9. In the presence of TGF-β and IL-15 but the absence of IL-4, strongly enhanced the expression of additional genes in Vδ2T high levels of IL-9 were secreted already 4 d after initial in vitro ac- cells, including IKAROS family zinc finger 4 (IKZF4; Eos), integrin tivation. -

Interleukin-9 Regulates Macrophage Activation in the Progressive Multiple Sclerosis Brain
Donninelli et al. Journal of Neuroinflammation (2020) 17:149 https://doi.org/10.1186/s12974-020-01770-z RESEARCH Open Access Interleukin-9 regulates macrophage activation in the progressive multiple sclerosis brain Gloria Donninelli1†, Inbar Saraf-Sinik1,2†, Valentina Mazziotti3, Alessia Capone1,4, Maria Grazia Grasso5, Luca Battistini1, Richard Reynolds6, Roberta Magliozzi3,6*† and Elisabetta Volpe1*† Abstract Background: Multiple sclerosis (MS) is an immune-mediated, chronic inflammatory, and demyelinating disease of the central nervous system (CNS). Several cytokines are thought to be involved in the regulation of MS pathogenesis. We recently identified interleukin (IL)-9 as a cytokine reducing inflammation and protecting from neurodegeneration in relapsing–remitting MS patients. However, the expression of IL-9 in CNS, and the mechanisms underlying the effect of IL-9 on CNS infiltrating immune cells have never been investigated. Methods: To address this question, we first analyzed the expression levels of IL-9 in post-mortem cerebrospinal fluid of MS patients and the in situ expression of IL-9 in post-mortem MS brain samples by immunohistochemistry. A complementary investigation focused on identifying which immune cells express IL-9 receptor (IL-9R) by flow cytometry, western blot, and immunohistochemistry. Finally, we explored the effect of IL-9 on IL-9-responsive cells, analyzing the induced signaling pathways and functional properties. Results: We found that macrophages, microglia, and CD4 T lymphocytes were the cells expressing the highest levels of IL-9 in the MS brain. Of the immune cells circulating in the blood, monocytes/macrophages were the most responsive to IL-9. We validated the expression of IL-9R by macrophages/microglia in post-mortem brain sections of MS patients. -

Cytokine Modulators As Novel Therapies for Airway Disease
Copyright #ERS Journals Ltd 2001 Eur Respir J 2001; 18: Suppl. 34, 67s–77s European Respiratory Journal DOI: 10.1183/09031936.01.00229901 ISSN 0904-1850 Printed in UK – all rights reserved ISBN 1-904097-20-0 Cytokine modulators as novel therapies for airway disease P.J. Barnes Cytokine modulators as novel therapies for airway disease. P.J. Barnes. #ERS Correspondence: P.J. Barnes Journals Ltd 2001. Dept of Thoracic Medicine ABSTRACT: Cytokines play a critical role in orchestrating and perpetuating National Heart & Lung Institute inflammation in asthma and chronic obstructive pulmonary disease (COPD), and Imperial College Dovehouse Street several specific cytokine and chemokine inhibitors are now in development for the future London SW3 6LY therapy of these diseases. UK Anti-interleukin (IL)-5 is very effective at reducing peripheral blood and airway Fax: 0207 3515675 eosinophil numbers, but does not appear to be effective against symptomatic asthma. Inhibition of IL-4 with soluble IL-4 receptors has shown promising early results in Keywords: Chemokine receptor asthma. Inhibitory cytokines, such as IL-10, interferons and IL-12 are less promising, cytokine as systemic delivery causes side-effects. Inhibition of tumour necrosis factor-a may be interleukin-4 useful in severe asthma and for treating severe COPD with systemic features. interleukin-5 interleukin-9 Many chemokines are involved in the inflammatory response of asthma and COPD interleukin-10 and several low-molecular-weight inhibitors of chemokine receptors are in development. CCR3 antagonists (which block eosinophil chemotaxis) and CXCR2 antagonists (which Received: March 26 2001 block neutrophil and monocyte chemotaxis) are in clinical development for the Accepted April 25 2001 treatment of asthma and COPD respectively. -

Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W
UPDATE ON GENE COMPLETIONS AND ANNOTATIONS Evolutionary divergence and functions of the human interleukin (IL) gene family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W. Nebert3* and Vasilis Vasiliou1 1Molecular Toxicology and Environmental Health Sciences Program, Department of Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO 80045, USA 2Department of Clinical Pharmacy, University of Colorado Denver, Aurora, CO 80045, USA 3Department of Environmental Health and Center for Environmental Genetics (CEG), University of Cincinnati Medical Center, Cincinnati, OH 45267–0056, USA *Correspondence to: Tel: þ1 513 821 4664; Fax: þ1 513 558 0925; E-mail: [email protected]; [email protected] Date received (in revised form): 22nd September 2010 Abstract Cytokines play a very important role in nearly all aspects of inflammation and immunity. The term ‘interleukin’ (IL) has been used to describe a group of cytokines with complex immunomodulatory functions — including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterisation. These molecules are under constant pressure to evolve due to continual competition between the host’s immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organisation. Within the literature there are a number of overlapping nomenclature and classification systems derived from biological function, receptor-binding properties and originating cell type. -
IL-9-Producing Invariant NKT Cells Protect Against DSS-Induced Colitis in an IL-4-Dependent Manner
nature publishing group ARTICLES See COMMENTARY page XX IL-9-producing invariant NKT cells protect against DSS-induced colitis in an IL-4-dependent manner H S K i m 1 a n d D H C h u n g 1 Although the T-helper type 9 (Th9) subset has recently been revisited, interleukin (IL)-9-producing invariant natural killer T (iNKT) cells remain poorly characterized. Moreover, whether IL-9-producing iNKT cells regulate colitis is unknown. Here, we investigated functions of IL-9-producing iNKT cells in dextran sulfate sodium (DSS)-induced colitis. Wild- type (WT) mice attenuated colitis compared to J 18 − / − mice, which were restored by the adoptive transfer of WT, but not IL-4-deficient iNKT cells. IL-4-deficient iNKT cells failed to produce IL-9, which was reversed by recombinant IL-4. Furthermore, iNKT cells, pre-incubated with anti-CD3 + CD28 monoclonal antibodies and IL-4 + tumor growth factor (TGF)- (IL-9 + iNKT), suppressed colitis in J 18 − / − mice, whereas pre-incubated IL-4-deficient iNKT cells did not. IL-9 blockade reversed IL-9 + iNKT cell-mediated colitis by increasing colonic IL-17A and interferon (IFN)- transcripts, but decreasing IL-9, IL-10, TGF- , PU.1, IFN regulatory factor 4, and signal transducer and activator of transcription 5 in J 18 − / − mice. In conclusion, IL-9-producing iNKT cells protect against DSS-induced colitis through IFN- and IL-17A suppression, but IL-10 and TGF- enhancement, depending on the IL-4 production by iNKT cells. INTRODUCTION recognize glycolipids presented by CD1d, a major histocom- Diverse CD4 + T-cell subsets regulate immune responses patibility complex (MHC) class I-like protein expressed by to transplantation, infection, tumor surveillance, and auto- antigen-presenting cells. -

Techniques for Immune Function Analysis Application Handbook 1St Edition
Techniques for Immune Function Analysis Application Handbook 1st Edition BD Biosciences For additional information please access the Immune Function Homepage at www.bdbiosciences.com/immune_function For Research Use Only. Not for use in diagnostic or therapeutic procedures. Purchase does not include or carry any right to resell or transfer this product either as a stand-alone product or as a component of another product. Any use of this product other than the permitted use without the express written authorization of Becton Dickinson and Company is strictly prohibited. All applications are either tested in-house or reported in the literature. See Technical Data Sheets for details. BD, BD Logo and all other trademarks are the property of Becton, Dickinson and Company. ©2003 BD Table of Contents Preface . 4 Chapter 1: Immunofluorescent Staining of Cell Surface Molecules for Flow Cytometric Analysis . 9 Chapter 2: BD™ Cytometric Bead Array (CBA) Multiplexing Assays . 35 Chapter 3: BD™ DimerX MHC:Ig Proteins for the Analysis of Antigen-specific T Cells. 51 Chapter 4: Immunofluorescent Staining of Intracellular Molecules for Flow Cytometric Analysis . 61 Chapter 5: BD FastImmune™ Cytokine Flow Cytometry. 85 Chapter 6: BD™ ELISPOT Assays for Cells That Secrete Biological Response Modifiers . 109 Chapter 7: ELISA for Specifically Measuring the Levels of Cytokines, Chemokines, Inflammatory Mediators and their Receptors . 125 Chapter 8: BD OptEIA™ ELISA Sets and Kits for Quantitation of Analytes in Serum, Plasma, and Cell Culture Supernatants. 143 Chapter 9: BrdU Staining and Multiparameter Flow Cytometric Analysis of the Cell Cycle . 155 Chapter 10: Cell-based Assays for Biological Response Modifiers . 177 Chapter 11: BD RiboQuant™ Multi-Probe RNase Protection Assay System . -

Human Cytokine Response Profiles
Comprehensive Understanding of the Human Cytokine Response Profiles A. Background The current project aims to collect datasets profiling gene expression patterns of human cytokine treatment response from the NCBI GEO and EBI ArrayExpress databases. The Framework for Data Curation already hosted a list of candidate datasets. You will read the study design and sample annotations to select the relevant datasets and label the sample conditions to enable automatic analysis. If you want to build a new data collection project for your topic of interest instead of working on our existing cytokine project, please read section D. We will explain the cytokine project’s configurations to give you an example on creating your curation task. A.1. Cytokine Cytokines are a broad category of small proteins mediating cell signaling. Many cell types can release cytokines and receive cytokines from other producers through receptors on the cell surface. Despite some overlap in the literature terminology, we exclude chemokines, hormones, or growth factors, which are also essential cell signaling molecules. Meanwhile, we count two cytokines in the same family as the same if they share the same receptors. In this project, we will focus on the following families and use the member symbols as standard names (Table 1). Family Members (use these symbols as standard cytokine names) Colony-stimulating factor GCSF, GMCSF, MCSF Interferon IFNA, IFNB, IFNG Interleukin IL1, IL1RA, IL2, IL3, IL4, IL5, IL6, IL7, IL9, IL10, IL11, IL12, IL13, IL15, IL16, IL17, IL18, IL19, IL20, IL21, IL22, IL23, IL24, IL25, IL26, IL27, IL28, IL29, IL30, IL31, IL32, IL33, IL34, IL35, IL36, IL36RA, IL37, TSLP, LIF, OSM Tumor necrosis factor TNFA, LTA, LTB, CD40L, FASL, CD27L, CD30L, 41BBL, TRAIL, OPGL, APRIL, LIGHT, TWEAK, BAFF Unassigned TGFB, MIF Table 1. -

UKPMC Funders Group Author Manuscript J Allergy Clin Immunol
UKPMC Funders Group Author Manuscript J Allergy Clin Immunol. Author manuscript; available in PMC 2012 June 28. Published in final edited form as: J Allergy Clin Immunol. 2012 April ; 129(4): 1000–10.e3. doi:10.1016/j.jaci.2011.12.965. UKPMC Funders Group Author Manuscript UKPMC Funders Group Author Manuscript Activin A and TGF-β promote TH9 cell–mediated pulmonary allergic pathology Carla P. Jones, PhD*, Lisa G. Gregory, PhD*, Benjamin Causton, BSc, Gaynor A. Campbell, PhD, and Clare M. Lloyd, PhD Leukocyte Biology Section, Faculty of Medicine, National Heart and Lung Institute, Imperial College, London, United Kingdom Abstract Background—IL-9-secreting (TH9) T cells are thought to represent a distinct T-cell subset. However, evidence for their functionality in disease is uncertain. Objective—To define a functional phenotype for TH9-driven pathology in vivo. Methods—We used fluorescence-activated cell sorting to identify circulating TH9 cells in atopic and nonatopic subjects. In mice we utilized a model of allergic airways disease induced by house dust mite to determine TH9 cell function in vivo and the role of activin A in TH9 generation. Results—Allergic patients have elevated TH9 cell numbers in comparison to nonatopic donors, which correlates with elevated IgE levels. In a murine model, allergen challenge with house dust mite leads to rapid TH9 differentiation and proliferation, with much faster kinetics than for TH2 cell differentiation, resulting in the specific recruitment and activation of mast cells. The TGF-β superfamily member activin A replicates the function of TGF-β1 in driving the in vitro generation of TH9 cells. -

An Update on Interleukin-9: from Its Cellular Source and Signal Transduction to Its Role in Immunopathogenesis
International Journal of Molecular Sciences Review An Update on Interleukin-9: From Its Cellular Source and Signal Transduction to Its Role in Immunopathogenesis Sushmita Chakraborty 1,* , Katharina F. Kubatzky 2 and Dipendra Kumar Mitra 1,* 1 Department of Transplant Immunology and Immunogenetics, All India Institute of Medical Sciences, New Delhi 1100029, India 2 Zentrum für Infektiologie, Medizinische Mikrobiologie und Hygiene, Universitätsklinikum Heidelberg, Im Neuenheimer Feld 324, 69120 Heidelberg, Germany; [email protected] * Correspondence: [email protected] (S.C.); [email protected] (D.K.M.); Tel.: +91-11-2659-4638 (D.K.M.) Received: 31 March 2019; Accepted: 24 April 2019; Published: 29 April 2019 Abstract: Interleukin-9 (IL-9) is a pleiotropic cytokine and was primarily studied in the context of T helper 2 (TH2)-associated immuno-pathological conditions such as asthma and parasitic infections. There was a paradigm shift in the biology of IL-9 after the recent discovery of TH9 cells, a new subtype of TH cells which secrete IL-9 in copious amounts. This has resulted in renewed interest in this cytokine, which was neglected since discovery because it was considered it to be just another TH2 cytokine. Recent studies have shown that it has multiple cellular sources and is critically involved in the immune-pathogenesis of inflammatory diseases and in guarding immune tolerance. In this review, we will discuss its discovery, gene organization, cellular sources, and signaling pathways. Especially, we will give an update on the recent development regarding its relevance in the immune pathogenesis of human diseases. Keywords: interleukin-9; inflammatory diseases; signal transduction 1. -
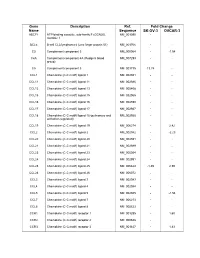
Supplementary Data
Gene Description Ref. Fold Change Name Sequence SK-OV-3 OVCAR-3 ABCF1 ATP-binding cassette, sub-family F (GCN20), NM_001090 - - member 1 BCL6 B-cell CLL/lymphoma 6 (zinc finger protein 51) NM_001706 - - C3 Complement component 3 NM_000064 - -1.54 C4A Complement component 4A (Rodgers blood NM_007293 - - group) C5 Complement component 5 NM_001735 13.74 - CCL1 Chemokine (C-C motif) ligand 1 NM_002981 - - CCL11 Chemokine (C-C motif) ligand 11 NM_002986 - - CCL13 Chemokine (C-C motif) ligand 13 NM_005408 - - CCL15 Chemokine (C-C motif) ligand 15 NM_032965 - - CCL16 Chemokine (C-C motif) ligand 16 NM_004590 - - CCL17 Chemokine (C-C motif) ligand 17 NM_002987 - - CCL18 Chemokine (C-C motif) ligand 18 (pulmonary and NM_002988 - - activation-regulated) CCL19 Chemokine (C-C motif) ligand 19 NM_006274 - 2.42 CCL2 Chemokine (C-C motif) ligand 2 NM_002982 - -2.23 CCL20 Chemokine (C-C motif) ligand 20 NM_004591 - - CCL21 Chemokine (C-C motif) ligand 21 NM_002989 - - CCL23 Chemokine (C-C motif) ligand 23 NM_005064 - - CCL24 Chemokine (C-C motif) ligand 24 NM_002991 - - CCL25 Chemokine (C-C motif) ligand 25 NM_005624 -1.49 2.59 CCL26 Chemokine (C-C motif) ligand 26 NM_006072 - - CCL3 Chemokine (C-C motif) ligand 3 NM_002983 - - CCL4 Chemokine (C-C motif) ligand 4 NM_002984 - - CCL5 Chemokine (C-C motif) ligand 5 NM_002985 - -1.54 CCL7 Chemokine (C-C motif) ligand 7 NM_006273 - - CCL8 Chemokine (C-C motif) ligand 8 NM_005623 - - CCR1 Chemokine (C-C motif) receptor 1 NM_001295 - 1.60 CCR2 Chemokine (C-C motif) receptor 2 NM_000648 - - CCR3 Chemokine (C-C -

Features and Roles of T Helper 9 Cells and Interleukin 9 in Immunological Diseases
Allergol Immunopathol (Madr). 2019;47(1):90---104 Allergologia et immunopathologia Sociedad Espa ˜nola de Inmunolog´ıa Cl´ınica, Alergolog´ıa y Asma Pedi ´atrica www.elsevier.es/ai REVIEW Features and roles of T helper 9 cells and interleukin 9 in immunological diseases a b b c b,∗ R. Yazdani , S. Shapoori , M. Rezaeepoor , R. Sanaei , M. Ganjalikhani-Hakemi , d,e f a a,g,h,∗ G. Azizi , W. Rae , A. Aghamohammadi , N. Rezaei a Research Center for Immunodeficiencies, Children’s Medical Center, Tehran University of Medical Science, Tehran, Iran b Department of Immunology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran c Department of Immunology, School of Medicine, Iran University of Medical Science, Tehran, Iran d Non-Communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran e Department of Laboratory Medicine, Imam Hassan Mojtaba Hospital, Alborz University of Medical Sciences, Karaj, Iran f Department of Immunology, MP8, University Hospital Southampton NHS Foundation Trust, Tremona Road, Southampton, Hampshire SO16 6YD, UK g Department of Immunology, School of Medicine, Tehran University of Medical Science, Tehran, Iran h Network of Immunity in Infection, Malignancy and Autoimmunity (NIIMA), Universal Scientific Education and Research Network (USERN), Tehran, Iran Received 19 November 2017; accepted 9 February 2018 Available online 25 April 2018 KEYWORDS Abstract T helper 9 (TH9) cells are considered as newly classified helper T cells that have an TH9 cells; important role in the regulation of immune responses. Since these cells preferentially produce IL-9; IL-9, these cells are termed TH9 cells. Recently, the role of TH9 and its signature cytokine (IL-9) Autoimmunity; has been investigated in a wide range of diseases, including autoimmunity, allergy, infections, Allergic; cancer and immunodeficiency. -

Interleukin 10 in Human Infectious Diseases
Review The two faces of interleukin 10 in human infectious diseases Jean-Louis Mege, Soraya Meghari, Amélie Honstettre, Christian Capo, Didier Raoult Resolution of infections depends on the host’s ability to mount a protective immune response. However, an Lancet Infect Dis 2006; 6: exacerbated response to infections may result in deleterious lesions. Consequently, immunoregulatory mechanisms 557–69 are needed to control immune response and prevent infection-associated lesions. Interleukin 10 may be a major Unité des Rickettsies, Centre regulator of innate and adaptive immunity in vitro and in animals, but its role in human infections is still unclear. National de la Recherche Scientifi que Unité Mixte de Review of the published work reveals wide involvement of interleukin 10 in two major features of infectious diseases. Recherche 6020, Institut On one hand, interleukin 10 prevents the development of immunopathological lesions that result from exacerbated Fédératif de Recherche 48, protective immune response to acute and chronic infections. On the other hand, it is critically involved in persistence Université de la Méditerranée, of bacteria and viruses by interfering with innate and adaptive protective immunity. Moreover, infections induce the Faculté de Médecine, Marseille, France (Prof J-L Mege MD, expansion of interleukin-10-producing regulatory cells that are involved in protection against allergic diseases. S Meghari PhD, A Honstettre PhD, C Capo PhD, Introduction inhibitory factor, and fi ve paralogues named interleukins Prof D Raoult MD) Resolution of infections depends on the host’s ability to 19, 20, 22 (interleukin-10-related T cell derived inducible Correspondence to: mount a protective immune response mainly based on factor), 24 (melanoma diff erentiation-associated Prof Didier Raoult, Unité des Rickettsies, Faculté de Médecine, 1,3 the recruitment of immunocompetent eff ectors and the antigen 7), and 26 (AK155).