PDF of Trial CTRI Website URL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thaltej Village: an Incremental Approach to Urban Encroachment
Thaltej Village: An Incremental Approach to Urban Encroachment Thaltej Village: An Incremental Approach to Urban Encroachment Emily Brown Allison Buchwach Ryan Hagerty Mary Richardson Laura Schultz Bin Yan Under the advisement of Professor Michael Dobbins Georgia Institute of Technology April 27, 2012 Acknowlegements This report was produced with help from faculty and students at CEPT University in Ahmdebad, as well as many other generous folks both here and abroad that have helped us immeasurably with their advice, insight and feedback along the way. To all, we extend our heartfelt gratitude. Contents 1 INTRODUCTION ............................................................................................................................................. 1 2 INDIAN NATIONAL CONTEXT ......................................................................................................................... 3 2.1 INDIA’S URBANIZATION AND ITS IMPACT ON SLUMS AND THE ENVIRONMENT ................................................................ 3 2.2 IMPACT OF URBANIZATION: ENVIRONMENTAL DEGRADATION .................................................................................... 5 2.3 POLICY RESPONSES ............................................................................................................................................ 6 2.4 POLICY RESPONSES ............................................................................................................................................ 8 2.4.1 Slum Clearance (1956) ............................................................................................................................ -

Ahmedabad Municipal Corporation Councillor List (Term 2021-2026)
Ahmedabad Municipal Corporation Councillor List (term 2021-2026) Ward No. Sr. Mu. Councillor Address Mobile No. Name No. 1 1-Gota ARATIBEN KAMLESHBHAI CHAVDA 266, SHIVNAGAR (SHIV PARK) , 7990933048 VASANTNAGAR TOWNSHIP, GOTA, AHMEDABAD‐380060 2 PARULBEN ARVINDBHAI PATEL 291/1, PATEL VAS, GOTA VILLAGE, 7819870501 AHMEDABAD‐382481 3 KETANKUMAR BABULAL PATEL B‐14, DEV BHUMI APPARTMENT, 9924136339 SATTADHAR CROSS ROAD, SOLA ROAD, GHATLODIA, AHMEDABAD‐380061 4 AJAY SHAMBHUBHAI DESAI 15, SARASVATINAGAR, OPP. JANTA 9825020193 NAGAR, GHATLODIA, AHMEDABAD‐ 380061 5 2-Chandlodia RAJESHRIBEN BHAVESHBHAI PATEL H/14, SHAYONA CITY PART‐4, NR. R.C. 9687250254, 8487832057 TECHNICAL ROAD, CHANDLODIA‐ GHATLODIA, AHMDABAD‐380061 6 RAJESHWARIBEN RAMESHKUMAR 54, VINAYAK PARK, NR. TIRUPATI 7819870503, PANCHAL SCHOOL, CHANDLODIA, AHMEDABAD‐ 9327909986 382481 7 HIRABHAI VALABHAI PARMAR 2, PICKERS KARKHANA ,NR. 9106598270, CHAMUDNAGAR,CHANDLODIYA,AHME 9913424915 DABAD‐382481 8 BHARATBHAI KESHAVLAL PATEL A‐46, UMABHAVANI SOCIETY, TRAGAD 7819870505 ROAD, TRAGAD GAM, AHMEDABAD‐ 382470 9 3- PRATIMA BHANUPRASAD SAXENA BUNGLOW NO. 320/1900, Vacant due to Chandkheda SUBHASNAGAR, GUJ. HO.BOARD, resignation of Muni. CHANDKHEDA, AHMEDABAD‐382424 Councillor 10 RAJSHRI VIJAYKUMAR KESARI 2,SHYAM BANGLOWS‐1,I.O.C. ROAD, 7567300538 CHANDKHEDA, AHEMDABAD‐382424 11 RAKESHKUMAR ARVINDLAL 20, AUTAMNAGAR SOC., NR. D CABIN 9898142523 BRAHMBHATT FATAK, D CABIN SABARMATI, AHMEDABAD‐380019 12 ARUNSINGH RAMNYANSINGH A‐27,GOPAL NAGAR , CHANDKHEDA, 9328784511 RAJPUT AHEMDABAD‐382424 E:\BOARDDATA\2021‐2026\WEBSITE UPDATE INFORMATION\MUNICIPAL COUNCILLOR LIST IN ENGLISH 2021‐2026 TERM.DOC [ 1 ] Ahmedabad Municipal Corporation Councillor List (term 2021-2026) Ward No. Sr. Mu. Councillor Address Mobile No. Name No. 13 4-Sabarmati ANJUBEN ALPESHKUMAR SHAH C/O. BABULAL JAVANMAL SHAH , 88/A 079- 27500176, SHASHVAT MAHALAXMI SOCIETY, RAMNAGAR, SABARMATI, 9023481708 AHMEDABAD‐380005 14 HIRAL BHARATBHAI BHAVSAR C‐202, SANGATH‐2, NR. -
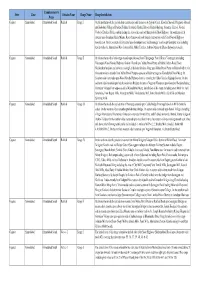
State Zone Commissionerate Name Division Name Range Name
Commissionerate State Zone Division Name Range Name Range Jurisdiction Name Gujarat Ahmedabad Ahmedabad South Rakhial Range I On the northern side the jurisdiction extends upto and inclusive of Ajaji-ni-Canal, Khodani Muvadi, Ringlu-ni-Muvadi and Badodara Village of Daskroi Taluka. It extends Undrel, Bhavda, Bakrol-Bujrang, Susserny, Ketrod, Vastral, Vadod of Daskroi Taluka and including the area to the south of Ahmedabad-Zalod Highway. On southern side it extends upto Gomtipur Jhulta Minars, Rasta Amraiwadi road from its intersection with Narol-Naroda Highway towards east. On the western side it extend upto Gomtipur road, Sukhramnagar road except Gomtipur area including textile mills viz. Ahmedabad New Cotton Mills, Mihir Textiles, Ashima Denims & Bharat Suryodaya(closed). Gujarat Ahmedabad Ahmedabad South Rakhial Range II On the northern side of this range extends upto the road from Udyognagar Post Office to Viratnagar (excluding Viratnagar) Narol-Naroda Highway (Soni ni Chawl) upto Mehta Petrol Pump at Rakhial Odhav Road. From Malaksaban Stadium and railway crossing Lal Bahadur Shashtri Marg upto Mehta Petrol Pump on Rakhial-Odhav. On the eastern side it extends from Mehta Petrol Pump to opposite of Sukhramnagar at Khandubhai Desai Marg. On Southern side it excludes upto Narol-Naroda Highway from its crossing by Odhav Road to Rajdeep Society. On the southern side it extends upto kulcha road from Rajdeep Society to Nagarvel Hanuman upto Gomtipur Road(excluding Gomtipur Village) from opposite side of Khandubhai Marg. Jurisdiction of this range including seven Mills viz. Anil Synthetics, New Rajpur Mills, Monogram Mills, Vivekananda Mill, Soma Textile Mills, Ajit Mills and Marsdan Spinning Mills. -

AMDHY Fact Sheet
NEAR VASTRAPUR LAKE, VASTRAPUR Ahmedabad, Gujarat 380015 India T + (9179) 6160 1234 F + (9179) 6160 1235 [email protected] ahmedabad.hyatthotels.hyatt.com you’re more than welcome 2013.02 Accommodations Services & Facilities • 178 rooms including 10 suites • 24-hour in-room dining • Size of the rooms range from 26 to 111.48 square metres • Airport limousine / limousine for hire (280 to 1,200 square feet) • Car rental • Choice of smoking and non-smoking rooms • 24-hour Assistant Manager / Concierge • Ergonomically designed beds • Car parking with valet parking facilities • Range of comfort pillows • Doctor on call • Bedside electronic controls • Currency exchange • Spacious bathroom with a walk-in shower cubicle • Executive Lounge for Executive room and Suite room guests (bath tub facility in Hyatt executive Suite only) • Bathroom amenities and hairdryer Conferences & Banquets • Tea- and coffee- making facilities • State-of-the-art conference and banquet facilities ideal for • Stocked minibar (optional health minibar) board meetings, closed door discussion, break away functions, • Individually controlled air-conditioning and lighting weddings and banquets are available at lobby level with • Large bay windows 1,068.39 square metres (11,500 square feet) of space • 32-inch LCD television with international channels • A pre-function area and two separate meeting rooms (40-inch LCD television in Suite Rooms) • Broadband data port / wired and wireless Internet accommodating smaller meetings • Iron / ironing board on request • Meeting areas are equipped with the latest software, Wi- • In-room electronic safes conferencing capabilities Recreational Facilities Restaurant, Bar & Lounge • Spa — featuring seven beautifully appointed rooms with • Collage — designed to be an uplifting yet informal experience, interconnecting shower cubicles, including a salon. -

Special Report on Ahmedabad City, Part XA
PRG. 32A(N) Ordy. 700 CENSUS OF INDIA 1961 VOLUME V GUJARAT PAR T X-A (i) SPECIAL REPORT ON AHMEDABAD CITY R. K. TRIVEDI Superintendent of Census Operations, Gujarat PRICE Rs. 9.75 P. or 22 Sh. 9 d. or $ U.S. 3.51 CENSUS OF INDIA 1961 LIST OF PUBLICATIONS CENTRAL GOVERNMENT PUBLICATIONS Census of India, 1961 Volume V-Gujarat is being published in the following parts: * I-A(i) General Report * I-A(ii)a " * I-A(ii)b " * I-A(iii) General Report-Economic Trends and Projections :\< I-B Report on Vital Statistics and Fertility Survey .\< I-C Subsidiary Tables -'" II-A General Population Tables * II-B(l) General Economic Tables (Tables B-1 to B-IV-C) * II-B(2) General Economic Tables (Tables B-V to B-IX) * II-C Cultural and Migration Tables :l< III Household Economic Tables (Tables B-X to B-XVII) * IV-A Report on Housing and Establishments * IV-B Housing and Establishment Tables :\< V-A Tables on Scheduled Castes and Scheduled Tribes V-B Ethnographic Notes on Scheduled Castes and Scheduled Tribes (including reprints) ** VI Village Survey Monographs (25 Monographs) VII-A Selected Crafts of Gujarat * VII-B Fairs and Festivals * VIII-A Administration Report-Enumeration " ~ N ~r£br Sale - :,:. _ _/ * VIII-B Administration Report-Tabulation ) :\' IX Atlas Volume X-A Special Report on Cities * X-B Special Tables on Cities and Block Directory '" X-C Special Migrant Tables for Ahmedabad City STATE GOVERNMENT PUBLICATIONS * 17 District Census Handbooks in English * 17 District Census Handbooks in Gl~arati " Published ** Village Survey Monographs for SC\-Cu villages, Pachhatardi, Magdalla, Bhirandiara, Bamanbore, Tavadia, Isanpur and Ghclllvi published ~ Monographs on Agate Industry of Cam bay, Wood-carving of Gujarat, Patara Making at Bhavnagar, Ivory work of i\1ahllva, Padlock .i\Iaking at Sarva, Seellc l\hking of S,v,,,-kundb, Perfumery at Palanpur and Crochet work of Jamnagar published - ------------------- -_-- PRINTED BY JIVANJI D. -

Bharatiya Janata Party – Gujarat State
BHARATIYA JANATA PARTY – GUJARAT STATE STATE DEPARTMENTS (ç±|¢¢x¢) - 2017 SR. DEPARTMENT NAMES MOBILE NO. NO. NAMES 9898000098 SHRI KAUSHIKBHAI PATEL [email protected] 9978405844 DEPARTMENT FOR 9427306026 GOOD GOVERNANCE, SHRI I. K. JADEJA [email protected] 1 9978405808 AND CENTER - STATE 9825005335 SHRI JAYNARAYANBHAI VYAS [email protected] COORDINATION 9978405316 [email protected], SHRI NARHARIBHAI AMIN 9825006667 [email protected] 9824212033 SHRI KAMLESHBHAI JOSHIPURA [email protected] 9978407041 [email protected], DEPARTMENT FOR SHRI KANUBHAI MAVANI 9825144801 [email protected] 2 POLICY RESEARCH [email protected], SHRI PRAKASHBHAI SONI 9825215092 [email protected] SHRI DR. JIGISHABEN SHETH (VADODARA 9825802410 [email protected] CITY) SHRI DR. HARSHAD PATEL 9426015397 [email protected] [email protected], SHRI RAJUBHAI DHRUV 9426719555 [email protected] 3 MEDIA DEPARTMENT [email protected], SHRI DR. HEMANTBHAI BHATT 9825010940 [email protected] 9033455797 [email protected], SHRI VINODBHAI JAIN (SURAT CITY) 9925118094 [email protected] MEDIA RELATIONS SHRI BHUPENDRASINH CHUDASMA 9909018303 [email protected] 4 DEPARTMENT SHRI BHARATBHAI PANDYA 9825010329 [email protected] [email protected], SHRI BHARGAVBHAI BHATT 9825802229 [email protected] TRAINING SHRI K. C. PATEL 9824063260 [email protected] 5 DEPARTMENT [email protected], SHRI RAJESHBHAI PATEL 9426500757 [email protected] SHRI PRADIPBHAI -

Linking Urban Lakes
LINKING URBAN LAKES: Assessment of Water Quality and its Environmental Impacts AKSHAY ANAND February, 2014 SUPERVISORS: Ir. M.J.G. Mark Brussel Ms. M. Kuffer LINKING URBAN LAKES: Assessment of Water Quality and its Environmental Impacts AKSHAY ANAND Enschede, The Netherlands, [February, 2014] Thesis submitted to the Faculty of Geo-Information Science and Earth Observation of the University of Twente in partial fulfilment of the requirements for the degree of Master of Science in Geo-information Science and Earth Observation. Specialization: Urban Planning and Management SUPERVISORS: Ir. M.J.G. Mark Brussel Ms. M. Kuffer THESIS ASSESSMENT BOARD: Ms. Prof. dr. ing. P.Y. Georgiadou Dr. ir. C.M.M. Mannaerts [ University of Twente ] DISCLAIMER This document describes work undertaken as part of a programme of study at the Faculty of Geo-Information Science and Earth Observation of the University of Twente. All views and opinions expressed therein remain the sole responsibility of the author, and do not necessarily represent those of the Faculty. ABSTRACT Lakes in urban and peri-urban areas are an important interface between planning and ecology, which demands environmentally responsive strategies, acknowledging problems like flooding, water pollution, and water quality with their complexities in design and engineering. The present study attempts to investigate the impacts of hydrological planning interventions on lake ecosystems. The research highlights the issues in experimental projects like ‘lake linking project’ carried out by Ahmedabad Urban Development Authority (AUDA). The integration of storm water infrastructure and lake ecosystem creates adverse pressure on lake water quality which is subsequently also transferred to other connected lakes. -
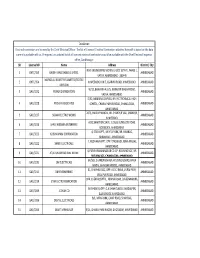
Portal Electrical Contactor List.Xlsx
Disclaimer: Electrical contractors are licenced by the Chief Electrical Officer. The list of Licenced Electrical Contractors attached herewith is based on the data currently available with us. If required, an updated list of all licenced electrical contractors would be available with the Chief Electrical Inspector office, Gandhinagar. SR License NO Name Address District / City R.M. ENGINEERING WORKS 3, GIDC ESTATE, PHASE 1, 1 GHTC/359 RAJESH MALLOABLES LIMITED. AHMEDABAD VATVA, AHMEDABAD - 382445 MAFATLAL INDUSTRIES LIMITED (TEXTILE 2 GHTC/354 AHMEDABAD UNIT, ASARWA ROAD, AHMEDABAD. AHMEDABAD DIVISION) W/12, BHAVANA FLATS, NARAYAN NAGAR ROAD, 3 GAC/3232 POWER DISTRIBUTORS AHMEDABAD VASNA, AHMEDABAD. D/92, NIRMAN COMPLEX, NR. R.C.TRCHNICAL HIGH 4 GAC/3228 PUSHYA ASSOCIATES SCHOOL, CHANAKYAPURI ROAD, CHANDLODIA, AHMEDABAD AHMEDABAD. 2675, HAIDAR MANZIL, NR. SHAHPUR VAD, SHAHPUR, 5 GAC/3227 SIGMA ELECTRIC WORKS AHMEDABAD AHMEDBAD. A/51,SHAKTI ENCLAVE, JUDGES BUNGLOW ROAD, 6 GAC/3226 SHREE KRISHNA ENTERPRISE AHMEDABAD BODEKDEV, AHMEDABAD. 4, STAR APPT., MEHTA PARK, NR. HIRABAG, 7 GAC/3225 ADISHVARAM CORPORATION AHMEDABAD AMBAWADI, AHMEDABAD. 7, NILDHARA APPT, OPP. VYASWADI, NAVA WADAJ, 8 GAC/3222 SHREE ELECTRICALS AHMEDABAD AHMEDABAD. 4, PARIBHRAHAMNAGAR CO.OP. HOUSING SOC, NR. 9 GAC/3221 ATULKUMAR RASIKLAL NAYAK AHMEDABAD RUTURAJ SOC, CHANDLODIA, AHMEDABAD. 64/583, CHANDRABHAGA HOUSING BOARD, NAVA 10 GAC/3220 OM ELECTRICALS AHMEDABAD WADAJ, BHAVSAR HOSTEL, AHMEDABAD. 51, SHAIWALI SOC, OPP. A.D.C. BANK, JIVRAJ PARK, 11 GAC/3215 OM ENGINEERING AHMEDABAD VEJALPUR ROAD, AHMEDABAD. OPP. CHEPI HOSPITAL, PIRANA ROAD, BAHERAMPURA, 12 GAC/3214 STAR ELCTRO FEBRICATION AHMEDABAD AHMEDABAD. AVISHKAR II, OPP. G.A.SHAH CLASSES, MADALPUR, 13 GAC/3209 JCSHAH CO AHMEDABAD ELLIS BRIDGE,AHMEDABAD. -
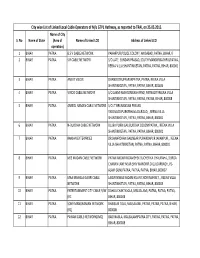
City Wise List of Linked Local Cable Operators of M/S GTPL Hathway, As Reported to TRAI, on 25.05.2015
City wise List of Linked Local Cable Operators of M/s GTPL Hathway, as reported to TRAI, on 25.05.2015. Name of City S. No Name of State (Area of Name of Linked LCO Address of Linked LCO operation) 1 BIHAR PATNA B.S.V CABLE NETWORK PAHARPUR,POLICE COLONY, ANISABAD, PATNA, BIHAR, 0 2 BIHAR PATNA S.R CABLE NETWORK S/O LATE. SUNDAR PRASAD, SOUTH MANDIRIKATHPULPATNA, REENA VILLA SHANTINIKETAN, PATNA, PATNA, BIHAR, 800001 3 BIHAR PATNA ANIKIT VISION DHANOOTRUPASPURPATNA, PATNA, REENA VILLA SHANTINIKETAN, PATNA, PATNA, BIHAR, 801506 4 BIHAR PATNA VINOD CABLE NETWORK S/O LAXMI MAHTONIKASH PIND, PATNACITYREENA VILLA SHANTINIKETAN, PATNA, PATNA, PATNA, BIHAR, 800008 5 BIHAR PATNA ANMOL NAMAN CABLE NETWORK S/O LT.BRIJNANDAN PRASAD YADAVLODIPURKESHAVLAALROAD, , REENA VILLA SHANTINIKETAN, PATNA, PATNA, BIHAR, 800001 6 BIHAR PATNA N‐BUDDHA CABLE NETWORK DUJRA PURBI GALIBUDDHA COLONYPATNA, , REENA VILLA SHANTINIKETAN, PATNA, PATNA, BIHAR, 800001 7 BIHAR PATNA RANJAN ENTERPRISES DR.JANARDHAN GALINEAR PURANDAPUR JAKANPUR, , REENA VILLA SHANTINIKETAN, PATNA, PATNA, BIHAR, 800001 8 BIHAR PATNA M/S MADAN CABLE NETWORK PATNA NAGAR NIGAMSHEK BUCHER KA CHAURAHA, DURGA CHARAN LANE NEAR SHIV MANDIRP.O GULZARBAGH, PS‐ ALAM GUNJ PATNA, PATNA, PATNA, BIHAR, 800007 9 BIHAR PATNA MAA MANGLA GAURI CABLE LAXMI NIWAS KADAM KUAN CHORI MARKET, , REENA VILLA NETWORK SHANTINIKETAN, PATNA, PATNA, BIHAR, 800003 10 BIHAR PATNA ENTERTAINMENT CITY CABLE N/W DAHUA CHAK NAGLA, MALSALAMI, PATNA, PATNA, PATNA, (RC BIHAR, 800008 11 BIHAR PATNA SONY MANORANJAN NETWORK BHAISANI TOLA, MALSALAMI, PATNA, PATNA, PATNA, BIHAR, (RC) 800008 12 BIHAR PATNA PAWAN CABLE NETWORK(JMD) BADI NAGLA, MALSALAMIPATNA CITY, PATNA, PATNA, PATNA, BIHAR, 800008 City wise List of Linked Local Cable Operators of M/s GTPL Hathway, as reported to TRAI, on 25.05.2015. -

Life Membership 2011-18 Dec Telephone
LIFE MEMBERSHIP 2011-18 DEC TELEPHONE . Sl.No Name Designation ADDRESS NO SABZAZRE_NASEEBA-17,Muslim 1 A A MUNSHI HD Pharmasist soc. Navrangpura, AHMEDABAD 9979148439 380009. Gineshwar part - I Nr Kanti part 2 A B PANT EE(D) society,Ghatlodia Ahmedabad- 27604204 380061 B-32, Orchid park nr Shailby Hospital 3 A C Bajaj Mgr(Logistic) 9904981023 opp. Karnavati c;lub Satelite , A-22,Park Avenue New cg 4 A C Barua Dy.SE(P) 9427336696 roadChandkheda Ahmedabad-380005 22,Somvil Bunglows,Bhaikaka Nagar 5 A C Saini SE(P) Thaltej Ahmedabad-55 B-302.Chinubhai Tower Satelite 6 A D PATEL SE(P) 9428563893 Memnagar AHMEDABAD-52. 27,Konark Society Sabarmati 7 A D VAID DySE(E) 9898218428 Ahmedabad -380019 A-9 AL-Ashurfi Society,B/H Haider 8 A G SHAIKH AEE(P) Nagar, JUHAPAURA, Sarkhej Road 9428330591 AHMEDABAD 380055 B-27, Shardakrupa Society, B/H 9 A H Naik Dy. SE(P) Janatanagar Chandkheda, 27516085 GANDHINAGAR-382424. 66/7 Madini Chamber ,Mahakali 10 A I Shaikh AE(M) Temple Dudeshwar Shahibaug 9824591030 Ahmedabad 8 Sindhu Mahal soc. Ashram road Old 11 A J Sharma DM(HR) 9428008152 Vadaj Ahmedabad 380013. D-303 Aditya residency Motera 12 A K Dhawan GM(Res) 9428332121 Ahmedabad 380005. H-6,Karnavati Soc.GHB Chandkheda 13 A K GAHLAUT GM(P) 23296926 Ahmedabad-382424 Flat no 1001 Sangath Diomond 14 A K Gupta Exe.Director Tower nr PVR cinema Motera 9712922825 Ahmedabad 380005. 2nd floor Rituraj Apartment op Rupal 15 A K Gupta DGM(MM) flats nr Xavier Loyla school 9426612638 Ahmedabad B-77,RJESHWARI 09428330135- 16 A K MEHTA EE(M) SOCIETY,PO,TRAGAD,IOC ROAD 27508082 CHANDKHEDA AHMEDABAD-382470. -
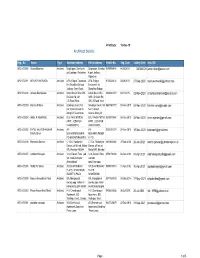
Architect Details
Print Date : 16-Dec-19 Architect Details Reg. No. Name Type Resident Address Office Address Mobile No. Reg. Date Validity Date Email ID ARCH/00290 Arvind Bhansali Architect Singh pole, Sarafon ki Singh pole, Sarafon 9099995495 04/08/2015 03/08/2020 [email protected] gali, Jodhpur, Rajasthan ki gali , Jodhpur, Rajasthan ARCH/00291 KEYUR R MEWADA Architect A/78, Falgun Tenament, A/78, Falgun 9725324420 28/09/2015 27-Sep-2020 [email protected] Nr. Shradhha School, Tenament, Nr. Jodhpur Gam Road, Shradhha School, ARCH/00292 Amruta Deshpande Architect A-604,David Villa CHS , A-604,David Villa 9892343979 03/11/2015 02-Nov-2020 [email protected] Dr.Dalvi Rd, Off CHS , Dr.Dalvi Rd, J.S.Road, Near Off J.S.Road, Near ARCH/00293 Harsha S More Architect Grindlays Court,Flat Grindlays Court,Flat 9867587770 03-Nov-2015 02-Nov-2020 [email protected] No.10,Amrut Keshav No.10,Amrut Marg,Off Gurunanak Keshav Marg,Off ARCH/00294 AMAL P AGARWAL Architect 501, PANCHTIRTH 501, PANCHTIRTH 9375970045 03-Nov-2015 02-Nov-2020 [email protected] APPT, JODHPUR APPT, JODHPUR CHAR RASTA, CHAR RASTA, ARCH/00295 PATEL KALPESHKUMAR Architect AT- AT- 9033097374 20-Nov-2015 19-Nov-2020 [email protected] RAVAJIBHAI MOHANPURKAMPA, MOHANPURKAMP PO-MADAPURKAMPA, A, PO- ARCH/00296 Hanuman Zanwar Architect C 1304, Rustomjee C 1304, Rustomjee 9619444648 27-Jan-2016 26-Jan-2021 [email protected] Elanza, off link rd, Malad Elanza, off link rd, (W), Mumbai 400064 Malad (W), Mumbai ARCH/00297 siddharth bhayani Architect 12-A, Devani Flats, Lad 12-A, Devani Flats, 9558151808 02-Apr-2016 01-Apr-2021 [email protected] soc road,Vastrapur, Lad soc Ahmedabad road,Vastrapur, ARCH/00298 SUKETU SHAH Architect 301,SHATRUNJAY 301,SHATRUNJAY 9909700019 11-Apr-2016 10-Apr-2021 [email protected] FLATS, SHANTIKUNJ FLATS, SOCIETY, PALDI, SHANTIKUNJ ARCH/00299 Mayur Himmatbhai Patel Architect 9/A, Mangalpark 9/A, Mangalpark 9377760193 18-May-2016 17-May-2021 [email protected] society,opp. -

Sheetal Westpark Residency
https://www.propertywala.com/sheetal-westpark-residency-ahmedabad Sheetal Westpark Residency - Vastrapur, Ahme… Softening the hectic pace of life outside Sheetal Westpark Residency by Sheetal Infrastructure at the very prime location of Vastrapur in Ahmedabad offers residential project that host 3 and 4 bhk apaprtments in various sizes. Project ID: J118951051 Builder: Sheetal Infrastructure Location: Sheetal Westpark Residency, Vastrapur, Ahmedabad - 380052 (Gujarat) Completion Date: Jun, 2022 Status: Started Description Sheetal Westpark Residency by Sheetal Infrastructure at the very prime location of Vastrapur in Ahmedabad offers residential project that host 3 and 4 bhk apartments in the size ranges in between 1585 to 2415 sqft. The project is well-equipped with lifestyle amenities such as cycling and jogging track, gymnasium, meditation room etc. Since the property is located in a prime location, schools, banks, ATMs and malls are all in the neighborhood. There are bus stops are within a radius of 2 kilometres of the project location. Amenities: Swimming Pool 24 X 7 Security Power Backup Landscape Garden and Tree Planting Car Parking Lift Available Gymnasium Children's play area Club House Intercom Jogging Track Library Recreation Facilities Skating Rink Movie Theater Air Hockey Chess Table Tennis Yoga & Meditation Area Pool & Snooker Carom Dance Hall Lawn Theater Secured And Gated Community Piped Gas Supply Sheetal Infrastructure began its journey in the real estate sector with its flagship project being a residential project ?Vedika Exotica?. Sheetal Infrastructure has become a renowned name in real estate development with an array of world-class projects. Our unprecedented success has a surprisingly simple explanation - consistently high performance and a strong commitment to customer service has attracted and retained more clients and project opportunities every year.