Vital Dyes for Staining Intraocular Membranes and Tissues During Vitrectomy an Overview of Vital Dyes and Their Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bilateral Same-Day Laser Peripheral Iridotomy in the Philadelphia Glaucoma Detection and Treatment Project
ORIGINAL STUDY Bilateral Same-day Laser Peripheral Iridotomy in the Philadelphia Glaucoma Detection and Treatment Project Michael Waisbourd, MD,* Anousheh Shafa, BS,* Radha Delvadia, BS,* Harjeet Sembhi, MPH,* Jeanne Molineaux, COA,* Jeffery Henderer, MD,w Laura T. Pizzi, PharmD, MPH,z Jonathan S. Myers, MD,* Lisa A. Hark, PhD, RD,* and L. Jay Katz, MD* reported in 2 patients (3%) and glare in 1 patient (1.5%). Thirteen Purpose: To report the outcomes of bilateral, same-day laser patients (19.7%) had repeat LPI treatment. All patients success- peripheral iridotomy (LPI) in the Philadelphia Glaucoma Detec- fully tolerated LPI treatment without serious complications. tion and Treatment Project. Conclusions: Performing bilateral, same-day LPI was well tolerated Methods: The Philadelphia Glaucoma Detection and Treatment in a large community-based, glaucoma detection and treatment Project was a community-based initiative aimed to improve project. Applying this treatment strategy may be considered in detection, management, treatment, and follow-up care of individ- similar settings, where patients’ access to eye care is limited and it uals at high risk for glaucoma. This novel project performed LPI, may be a cost-effective strategy. where 2 eyes received laser therapy on the same day. Of the 1649 patients examined between January 1, 2013 and May 31, 2014, Key Words: glaucoma detection, angle closure, laser peripheral patients who underwent bilateral, same-day LPI were included in iridotomy, bilateral same-day ocular procedures our analysis. Main outcome measures were visual acuity, intra- (J Glaucoma 2016;25:e821–e825) ocular pressure (IOP), and postoperative complication rates. Results: A total of 132 eyes of 66 patients underwent bilateral, aser iridotomy is aimed to eliminate the relative pupil- same-day LPI. -

Special Considerations in Cataract Surgery: Five Cornea Challenges
Clinical Update EXTRA CONTENT AVAILABLE CATARACT Special Considerations in Cataract Surgery: Five Cornea Challenges by linda roach, contributing writer interviewing preston h. blomquist, md, rosa a. braga-mele, md, kimberly a. drenser, md, phd, herbert e. kaufman, md, marguerite mcdonald, md, and roger f. steinert, md s the most common surgical choices, said Marguerite McDonald, IOL Selection procedure in ophthalmol- MD, of Lynbrook, N.Y. The device en- ogy, replacement of a cloudy ables the surgeon to directly measure 1 crystalline lens with an the eye’s aphakic refractive power in intraocular lens (IOL) usu- the operating room. Aally presents the ophthalmologist with Using intraoperative aberrometry is familiar sets of surgical routines. But becoming more commonplace, as “it what about those cases that involve may help in achieving more accuracy comorbidities or other complicating with IOL power selection,” Dr. Mc- factors? Donald said. Several experts shared their per- Tips on IOL selection. The chosen spectives on approaching out-of-the- IOL should be shaped to neutralize After an off-center LASIK procedure ordinary cataract surgeries in ways spherical aberrations, said Rosa A. such as this, the irregularity of the that offer the best chance at optimiz- Braga-Mele, MD, at the University of corneal topography indicates that a ing patient outcomes. This month, Toronto. “In anybody who has had multifocal IOL should be avoided. here’s a look at five challenges involv- myopic LASIK or PRK, I think it’s very ing the cornea. important to use a negatively aspheric prior to cataract surgery. 2) A dysfunc- IOL, because these patients have more tional, unstable tear film will affect the Challenge: Prior Refractive Surgery positively aberrant corneas. -

Clinical and Epidemiological Aspects of Cornea Transplant Patients of a Reference Hospital1
Rev. Latino-Am. Enfermagem Original Article 2017;25:e2897 DOI: 10.1590/1518-8345.1537.2897 www.eerp.usp.br/rlae Clinical and epidemiological aspects of cornea transplant patients of a reference hospital1 Giovanna Karinny Pereira Cruz2 Isabelle Campos de Azevedo2 Diana Paula de Souza Rego Pinto Carvalho2 Allyne Fortes Vitor3 Viviane Euzébia Pereira Santos3 Marcos Antonio Ferreira Júnior3 Objective: clinically characterizing cornea transplant patients and their distribution according to indicated and post-operative conditions of cornea transplantation, as well as estimating the average waiting time. Method: a cross-sectional, descriptive and analytical study performed for all cornea transplants performed at a reference service (n=258). Data were analyzed using Statistical Package for the Social Sciences, version 20.0. Results: the main indicator for cornea transplant was keratoconus. The mean waiting time for the transplant was approximately 5 months and 3 weeks for elective transplants and 9 days for urgent cases. An association between the type of corneal disorder with gender, age, previous surgery, eye classification, glaucoma and anterior graft failure were found. Conclusion: keratoconus was the main indicator for cornea transplant. Factors such as age, previous corneal graft failure (retransplantation), glaucoma, cases of surgeries prior to cornea transplant (especially cataract surgery) may be related to the onset corneal endothelium disorders. Descriptors: Corneal Transplantation; Corneal Diseases; Health Profile. 1 Paper extracted from Master’s Thesis “Corneal transplants in Rio Grande do Norte state: epidemiological and clinical aspects”, presented to Universidade Federal do Rio Grande do Norte, Natal, RN, Brazil. Supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. -

Intraocular Pressure During Phacoemulsification
J CATARACT REFRACT SURG - VOL 32, FEBRUARY 2006 Intraocular pressure during phacoemulsification Christopher Khng, MD, Mark Packer, MD, I. Howard Fine, MD, Richard S. Hoffman, MD, Fernando B. Moreira, MD PURPOSE: To assess changes in intraocular pressure (IOP) during standard coaxial or bimanual micro- incision phacoemulsification. SETTING: Oregon Eye Center, Eugene, Oregon, USA. METHODS: Bimanual microincision phacoemulsification (microphaco) was performed in 3 cadaver eyes, and standard coaxial phacoemulsification was performed in 1 cadaver eye. A pressure transducer placed in the vitreous cavity recorded IOP at 100 readings per second. The phacoemulsification pro- cedure was broken down into 8 stages, and mean IOP was calculated across each stage. Intraocular pressure was measured during bimanual microphaco through 2 different incision sizes and with and without the Cruise Control (Staar Surgical) connected to the aspiration line. RESULTS: Intraocular pressure exceeded 60 mm Hg (retinal perfusion pressure) during both standard coaxial and bimanual microphaco procedures. The highest IOP occurred during hydrodissection, oph- thalmic viscosurgical device injection, and intraocular lens insertion. For the 8 stages of the phaco- emulsification procedure delineated in this study, IOP was lower for at least 1 of the bimanual microphaco eyes compared with the standard coaxial phaco eye in 4 of the stages (hydro steps, nu- clear disassembly, irritation/aspiration, anterior chamber reformation). CONCLUSION: There was no consistent difference in IOP between the bimanual microphaco eyes and the eye that had standard coaxial phacoemulsification. Bimanual microincision phacoemul- sification appears to be as safe as standard small incision phacoemulsification with regard to IOP. J Cataract Refract Surg 2006; 32:301–308 Q 2006 ASCRS and ESCRS Bimanual microincision phacoemulsification, defined as capable of insertion through these microincisions become cataract extraction through 2 incisions of less than 1.5 mm more widely available. -

Effects of Cataracts on Flicker Electroretinograms Recorded with Reteval™ System: New Mydriasis-Free ERG Device
Miura et al. BMC Ophthalmology (2016) 16:22 DOI 10.1186/s12886-016-0200-x RESEARCHARTICLE Open Access Effects of cataracts on flicker electroretinograms recorded with RETeval™ system: new mydriasis-free ERG device Gen Miura*, Yosuke Nakamura, Eiju Sato and Shuichi Yamamoto Abstract Background: The purpose of this study was to evaluate the effects of cataracts on the flicker electroretinograms (ERGs) recorded with the RETeval™ system under mydriatic-free conditions. Methods: This was a retrospective study of 82 eyes of 60 patients with cataracts and 52 eyes of 38 patients who were pseudophakic. Flicker ERGs were recorded with the RETeval™ system (LKC Technologies, Gaithersburg, MD) under mydriatic-free condition with skin electrodes. Flicker ERGs were elicited by white light delivered at a frequency of 28.3 Hz and intensity of 8 Td-s. The implicit times and amplitudes of the ERGs recorded from the Grade 2 cataract, Grade 3 cataract, and pseudophakic groups were compared. Results: The mean amplitude was significantly smaller in both cataract groups than the pseudophakic group (Grade 2 cataract vs pseudophakic group, P < 0.0001; Grade 3 cataract vs pseudophakic group, P < 0.0001; Grade 2 cataract vs Grade 3 cataract, P = 0.027). The mean implicit times was significantly longer in both cataract groups than the pseudophakic group (Grade 2 cataract vs pseudophakic group, P = 0.046; Grade 3 cataract vs pseudophakic group, P = 0.0004; Grade 2 cataract vs Grade 3 cataract, P = 0.0084). Conclusions: The results indicate that the presence of Grade 2 or more cataracts will affect both the amplitude and the implicit time of the flicker ERGs. -

Five-Year Outcomes of Trabeculectomy and Phacotrabeculectomy
Open Access Original Article DOI: 10.7759/cureus.12950 Five-Year Outcomes of Trabeculectomy and Phacotrabeculectomy Danny Lam 1 , David Z. Wechsler 1, 2 1. Ophthalmology, University of Sydney, Sydney, AUS 2. Ophthalmology, Macquarie University, Sydney, AUS Corresponding author: Danny Lam, [email protected] Abstract Purpose The purpose of this study is to examine five-year outcomes of trabeculectomy and compare the stand-alone procedure when combined with phacoemulsification. Patients and methods This study included 123 eyes of 109 patients, with 79 patients in the trabeculectomy group and 44 patients in the phacotrabeculectomy group. Non-randomized comparative cohort study with data collected retrospectively from an existing database compiled by a single surgeon operating in Sydney, Australia from 2007 to 2019. The primary outcome measure was intraocular pressure. Secondary outcome measures were a number of glaucoma medications, treatment success rates, best-corrected visual acuity, bleb morphology, post-operative complications, and re-operation rate. Results The mean intraocular pressure was 10.6 ± 2.7 mm Hg in the trabeculectomy group (pre-operative mean intraocular pressure of 28.0 ± 9.8) and 12.0 ± 3.0 mm Hg in the phacotrabeculectomy group (pre-operative mean intraocular pressure of 23.4 ± 7.9) after five years (P = 0.052). The number of glaucoma medications required was 0.3 ± 0.7 in the trabeculectomy group (pre-operative mean of 3.7 ± 1.1) and 1.3 ± 1.2 in the phacotrabeculectomy group (pre-operative mean of 3.1 ± 1.0, P < 0.001). Conclusions Intraocular pressure reduction post-operatively over five years was similar between trabeculectomy and phacotrabeculectomy as determined by mean intraocular pressure, and intraocular pressure reduction from baseline. -

Florida Board of Medicine and Florida Board Of
FLORIDA BOARD OF MEDICINE AND FLORIDA BOARD OF OSTEOPATHIC MEDICINE APPROVED INFORMED CONSENT FORM FOR CATARACT OPERATION WITH OR WITHOUT IMPLANTATION OF INTRAOCULAR LENS DOES THE PATIENT NEED OR WANT A TRANSLATOR, INTERPRETOR OR READER? YES _____ NO_____ TO THE PATIENT: You have the right, as a patient, to be informed about your cataract condition and the recommended surgical procedure to be used, so that you may make the decision whether or not to undergo the cataract surgery, after knowing the risks, possible complications, and alternatives involved. This disclosure is not meant to scare or alarm you; it is simply an effort to make you better informed so that you may give or withhold your consent to cataract surgery and should reflect the information provided by your eye surgeon. If you have any questions or do not understand the information, please discuss the procedure with your eye surgeon prior to signing. WHAT IS A CATARACT, AND HOW IS IT TREATED? The lens in the eye can become cloudy and hard, a condition known as a cataract. Cataracts can develop from normal aging, from an eye injury, various medical conditions or if you have taken certain medications such as steroids. Cataracts may cause blurred vision, dulled vision, sensitivity to light and glare, and/or ghost images. If the cataract changes vision so much that it interferes with your daily life, the cataract may need to be removed to try to improve your vision. Surgery is the only way to remove a cataract. You can decide to postpone surgery or not to have the cataract removed. -

Division of Health Care Financing & Policy SB 278 Section 16
Division of Health Care Financing & Policy SB 278 Section 16 - Physician Rates Reporting Facility & Non-Facility Rate Comparison Nevada 2016 2016 Medicaid Medicaid Medicaid Medicare Medicare vs. vs. Procedure Code & Description Rates (1) Non-Facility Facility Medicare Medicare Rates for Rates for Non-Facility Facility 10021 Fna w/o image 70.92 128.90 72.80 (57.98) (1.88) 10022 Fna w/image 65.39 148.21 68.38 (82.82) (2.99) 10030 Guide cathet fluid drainage 154.73 827.01 176.71 (672.28) (21.98) 10035 Perq dev soft tiss 1st imag 86.35 568.38 90.92 (482.03) (4.57) 10036 Perq dev soft tiss add imag 43.52 495.42 45.82 (451.90) (2.30) 10040 Acne surgery 87.47 106.03 92.10 (18.56) (4.63) 10060 Drainage of skin abscess 95.60 122.68 101.60 (27.08) (6.00) 10061 Drainage of skin abscess 178.04 215.50 188.01 (37.46) (9.97) 10080 Drainage of pilonidal cyst 103.29 188.83 107.87 (85.54) (4.58) 10081 Drainage of pilonidal cyst 172.45 281.57 177.64 (109.12) (5.19) 10120 Remove foreign body 103.22 159.56 108.73 (56.34) (5.51) 10121 Remove foreign body 185.58 287.08 194.44 (101.50) (8.86) 10140 Drainage of hematoma/fluid 118.06 170.87 124.18 (52.81) (6.12) 10160 Puncture drainage of lesion 95.62 136.49 100.72 (40.87) (5.10) 10180 Complex drainage wound 178.97 258.94 188.14 (79.97) (9.17) 11000 Debride infected skin 28.42 56.88 29.76 (28.46) (1.34) 11001 Debride infected skin add-on 14.22 22.40 14.87 (8.18) (0.65) 11004 Debride genitalia & perineum 579.35 608.28 608.28 (28.93) (28.93) 11005 Debride abdom wall 781.65 822.97 822.97 (41.32) (41.32) 11006 Debride -

Comprehensive Strategies for Unplanned Vitrectomy for the Anterior Segment Surgeon
Supplement to January 2012 When the Room Gets Quiet Comprehensive strategies for unplanned vitrectomy for the anterior segment surgeon. A monograph and DVD based on the live surgery course given by Lisa B. Arbisser, MD. PLUS: A DVD OF SURGICAL VIDEOS AND A DIGITAL VERSION AVAILABLE AT EYETUBE.NET When the Room Gets Quiet An Ounce of Prevention All ophthalmic surgeons must have a comprehensive strategy for managing surgical complications. Many opinions and recommendations exist for handling complicated cataract surgeries. This monograph contains mine. I was trained as a comprehensive ophthalmologist, and for many years I performed scleral buckles, tra- beculectomy, and penetrating keratoplasty before con- centrating on cataract surgery and anterior segment reconstruction. The strategies I have developed for man- aging complex and complicated cataracts over decades of practice are enhanced by close collaboration with retinal surgeons as well as laboratory exploration. I hope this information will help you to organize your thoughts and have a cogent strategy at hand for ‘when the room gets quiet.’ An unplanned vitrectomy. —Lisa B. Arbisser, MD To view a digital version of this monograph with the videos from the accompanying DVD as well as additional materials, visit http://www.Eyetube.net/unplanned-vitrectomy. Table of Contents 3 Preoperative Evaluation of Problematic Eyes 11 Rationale for a Pars Plana Incision 3 Preventing Complications 12 Vitrectomy Goals and Parameters 5 Early Stages of Complications 13 Getting Started 6 Stages of Complications -
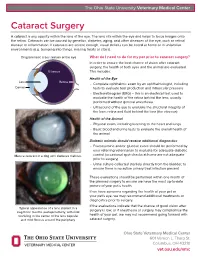
Cataract Surgery a Cataract Is Any Opacity Within the Lens of the Eye
The Ohio State University Veterinary Medical Center Cataract Surgery A cataract is any opacity within the lens of the eye. The lens sits within the eye and helps to focus images onto the retina. Cataracts can be caused by genetics, diabetes, aging, and other diseases of the eye, such as retinal disease or inflammation. If cataracts are severe enough, visual deficits can be noted at home or in unfamiliar environments (e.g. bumping into things, missing treats or stairs). Diagrammatic cross section of the eye What do I need to do for my pet prior to cataract surgery? In order to ensure the best chance of vision after cataract surgery, the health of both eyes and the animal are evaluated. Vitreous This includes: Health of the Eye Lens Retina • Complete ophthalmic exam by an ophthalmologist, including Cornea tests to evaluate tear production and intraocular pressure • Electroretinogram (ERG) – this is an electrical test used to evaluate the health of the retina behind the lens, usually performed without general anesthesia • Ultrasound of the eye to evaluate the structural integrity of the lens, retina and fluid behind the lens (the vitreous) Health of the Animal • Physical exam, including listening to the heart and lungs • Basic blood and urine tests to evaluate the overall health of the animal Diabetic animals should receive additional diagnostics • Fructosamine and/or glucose curve should be performed by your referring veterinarian to evaluate for adequate diabetic Mature cataract in a dog with diabetes mellitus. control (occasional spot-checks at home are not adequate prior to surgery) • Urine culture collected sterilely directly from the bladder, to ensure there is no active urinary tract infection present These evaluations should be performed within one month of the planned surgery to ensure we have the most up-to-date picture of your pet’s health. -

History of Cataract Surgery
History of Cataract Surgery A cataract is a clouding of the normally clear lens of the eye and can be addressed through a procedure that removes the affected lens and replaces it with a manmade lens known as an intraocular lens or IOL.1 Associated with improvements in vision, overall health, and cognitive and emotional well-being,2 cataract surgery is a safe and effective procedure1 and is the most commonly performed surgery in the world.3 One of the oldest surgical procedures known, cataract surgery was first documented in the fifth century BC.4 1747 French ophthalmologist Jacques Daviel is credited 1998 Toric IOLs are rolled out to correct astigmatism. Toric with the first Extracapsular Cataract Extraction, a IOLs have different powers in different meridians of the technique that uses a small incision to remove the lens and lens to correct the asymmetric power of the eye that is minimize the wound.5,6 characteristic of astigmatism.10 1753 London surgeon Samuel Sharp performs the first 2004 Aspheric IOLs, which closely match the shape and optical Intracapsular Cataract Extraction, a technique that quality of the eye’s natural lens, are introduced for sharper uses a large incision to remove the entire natural vision — especially in low light conditions and when the lens and capsule.7 pupil is dilated.11 1949 Intraocular lenses, manmade lenses made of 2010 Femtosecond laser is cleared by the FDA for cataract polymethylmethacrylate, are introduced by Sir Harold surgery.12 The femtosecond laser replaces or supports Ridley in London.8 Previously, -

Lensx Laser Cataract Surgery Implant
LenSx Laser Corneal Transplantation Cataract Surgery The cornea is the clear tissue on the front of your eye where light passes and is projected on the retina at the back of the eye. With disease or LenSx is a new laser technology allowing injury, the cornea can become cloudy. Corneal Alabama Vision Center is the leading provider completely blade-free cataract surgery. While transplantation, a common and successful of cataract and refractive surgical procedures in traditional cataract surgery offers excellent outpatient procedure, improves vision by Birmingham and the surrounding areas. Our outcomes: the LenSx laser customizes results for replacing the diseased cornea with a normal Board-Certified ophthalmologists, Dr. Price each eye, yielding superior visual outcomes. cornea. Kloess, Dr. Andrew Velazquez and Dr. Randall Cataract surgery involves replacing the clouded Descemet’s Stripping Automated Endothelial Pitts offer advanced surgical technologies lens in the eye with a new clear intraocular lens Keratoplasty (DSAEK) is a procedure in which the including traditional no-stitch and Lensx laser Cataract Surgery implant. There have been improvements in the unhealthy corneal tissue is removed and replaced cataract surgery, ReSTOR bifocal implants, laser treatment of cataracts that allow us to tailor the with healthy donor tissue. DSAEK utilizes a much glaucoma surgery, iLASIK blade-free all laser & Premium Lenses surgery to each patient. Some of these smaller surgical incision, requires fewer corneal custom LASIK, Implantable Contact Lenses, and innovations include new bifocal premium lenses sutures and allows for more rapid recovery and Corneal Transplant Surgery. Alabama Vision Cataracts occur when the natural lens becomes Center's knowledgeable staff is committed to clouded over time, interfering with normal to correct distance and near vision, and lenses to return to normal activities.