Wood Discolourations & Their Prevention
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deck Prep Sharkskin
General Deck Staining Tips NEW DECKS TIPS • Select dry lumber. If possible use kiln dried lumber with moisture content less than 16%. Paint the deck boards as soon as possible as checking and splitting can occur within days of exposure to warm dry air. • If staining your deck with 724 Series SharkSkin® solid hide stain, prime all 6 sides of each deck board with 15130 Alkyd Stain Blocking Primer prior to installation to prevent moisture ingress into the underside of the deck. Trimmed boards should be primed while still accessible. A ground-level moisture ® barrier beneath the deck is also recommended for grade or near grade level decks in damp areas. • New Cedar decks that will be stained to lighter colours must be primed with a coat of 15130 Alkyd or 05130 Acrylic Stain Blocking Primer to protect against tannin stain bleeding through the topcoat. • Woods such as Pine and Yellow Cedar that are prone to mill glaze should be treated with SharkSkin® 80319 Mill Glaze Remover (follow label directions) or SharkSkin aggressively sand using 60 grit paper. • If possible, install the deck boards so that the growth rings are arching up and not down so if cupping occurs, water will drain o the boards as opposed to ponding in the center of the board. • Pressure treated woods should be allowed to dry for 2 – 4 weeks prior to installation depending on temperature and humidity conditions preferably away from direct sunlight. Pre-prime all 6 sides as per above only if staining with 724 Series SharkSkin® solid hide stain. Priming is not required when using 725 Series SharkSkin® translucent wood stain. -

Wood Preparation
WOOD PREPARATION Penofin® Pro-Tech Stripper pecially developed to effectively remove oil • Fast acting Sfinishes on all exterior wood, composite or concrete surfaces using New Clean Strip Technology. • Easy to use Pro-Tech Stripper leaves little or no residue, will not • Removes paint, stains, sealers raise grain and provides double the coverage of average strippers. Pro-Tech Stripper is first step in preparing and loose wood fibers your wood for a beautiful Penofin application • Certified no VOC’s product Penofin® Pro-Tech Cleaner he best outdoor cleaner for just about everything, • Restores wood to mill Tthis unique formula uses Super Hydrogen Power to tackle just about any cleaning job around bright appearance your home. Mix these concentrated granules in • Cleans and rejuvenates wood varying strengths to effectively remove grease, grime, dirt, organic stains, tree sap and mineral deposits. • Easy to apply Kills mold and mildew. For use on wood, masonry, • Biodegradable; safe on soil concrete, fiber cement, fiberglass, outdoor furniture, and plant life cushions and floor coverings, glass and tile. Penofin® Pro-Tech Brightener nnihilates tough tannin stains and watermarks • The hardwood helper Aon all types of wood decking siding and fences; removes mill glaze. Penofin Pro-Tech Brightener • Reduces mill glaze for reestablishes the Ph balance of your wood after better penetration stripping and cleaning and brightens gray weathered • Removes the gray wood to bring back that mill-bright color. • Prepares hardwood for finishing Materials and tools for the project Before 16 WOOD PREPARATION WOOD PREPARATION BEFORE FINISHING Cleaning and preparing wood surface. There are variables to consider when preparing and finishing a Old wood: Old wood is more porous and may need more wood surface is the wood new, old or weathered? stain to cover the surface. -

Wood Finishes and Stains
T FEBRUARY 2005 WOOD FINISHES AND STAINS The overall market for wood finishes -- coatings which, unlike paint, do not completely mask the wood’s appearance -- has been grow- ing, along with the demand for environmentally preferable wood REPORfinishes. The demand for finishes for wood furniture, floors, and fixtures has increased from 82 million gallons to 100 million gallons over the last 5 years. About $2 billion in wood finishes were sold in 2004. his increased demand toxicity in household and building for wood coatings has products, manufacturers of wood T been driven by favorable coatings are offering many more housing markets fueled products that are low in by low interest rates, the volatile organic compounds trend toward larger, more (VOCs) and formulated expensive homes, and an without aromatic solvents, increase in homeowners heavy metals, or cancer- improving and redecorating causing chemicals. These their dwellings, inside and products are healthier for out. At the same time, the users of these products decorating trends that focus – namely, workers on new on the warmth and beauty of home construction projects and wood in the form of exposed wood do-it-yourself homeowners – as floors, walls, and home well as the occupants of furniture increase the Wood-finish manu- buildings where these demand for wood finishes facturers have also finishing products have as alternatives to paint. been applied. Wood- stepped up the pro- finish manufacturers Many of the products duction of “green” have also stepped up the used to coat wood products because of production of “green” around the home have products because of the the rising interest traditionally contained rising interest among volatile organic solvents among developers developers in obtaining and other toxic chemicals in obtaining Leader- Leadership in Energy and SEAL’S that have been linked to Environmental Design ship in Energy and poor indoor air quality (LEED) certification for and possible health Environmental new buildings. -

The Guide to Owning Heart Pine: a Rare American Treasure
The Guide to Owning Heart Pine: A Rare American Treasure OODWIOODWI GG Heart Pine Company NN Some Beginning Words The information provided in this booklet is just the beginning of what you need to know about fine wood floors. Since we do not know all of the special conditions in your home, it is not pos- sible to meet all of your information needs here. Due to the vast amount of technical considerations for installing a wood floor, we recom- mend consulting a wood flooring professional. Goodwin Heart Pine would be glad to help locate someone in your area. If we can help with additional information needs, we are glad to do so. Please tell us about your experiences so we can pass them along to future Heart Pine owners. We truly appreciate your interest in Heart Pine and Heart Cypress. Copyright © Goodwin Heart Pine Company, Inc,, 2001. All rights reserved. INTRODUCTION Retrieving the Past 1 Listening to Mother Nature 2 Why choose wood floors? HEART PINE HISTORY Heart Pine: A Rare American Treasure 3 A Walk Through Time 5 Growing-Oh-So-Great 6 Planting for the Future 7 Restoring The Longleaf for future generations INSTALLING AND FINISHING This Way to the Finish 10 Nine installation and finishing essentials The Inside Story on Site Conditions 12 Getting down the installation basics Finishing Heart Pine has Never Been Easier 16 Our step-by-step suggestions for finishing Heart Pine MAINTENANCE AND FLOOR RESTORATION Keeping Your Floors Looking Beautiful 21 Simple steps to preventive maintenance Reviving Your Floors' Finish 22 When it's time to -

Wood Stain Technical Data Sheet Octorber 2017 Page 1/2
Wood Stain Technical Data Sheet Octorber 2017 Page 1/2 » protects and maintains half-timbered construction, balcony cladding and other outdoor wood » weatherproof surface » water- and dirt-repellent » block resistance » on natural oil basis Product characteristics: All wood in the garden: Transparent wood stain on natural oil base for all outdoor wood pergolas, screen panels, trellis, fences, garden gates, garden with a highly weatherproof surface. SAICOS Wood Stain provides houses, … a beautiful natural colouration of the wood. Open-pored, Perfect for pressure impregnated wood. Suitable for thermo- water-repellent and extremely durable. Reduces swelling and wood (trial application recommended). shrinking of the wood. Natural oils penetrate deeply into the wood and protect effectivelly against moisture and weather Preparation: influences. Colour pigments with high UV-resistance ensure Wood surface must be dry and clean (max. moisture content durable beauty of the wood surface. 18%). Remove old varnishes. Previously with SAICOS Wood Stain SAICOS Wood Stain bonds permanently with the wood – there- or other open-pored stains treated wood needs only to be cle- fore no sanding in case of renovation, not even after many years. aned, sanding not required. The final shade of transparent stains is influenced by the wood background – you might make Colours: a trial coating. If possible coat untreated wood on all sides prior 0001 Colourless transparent installation. 0009 White transparent Wood extremely exposed to weather influences can be protected 0010 Spruce transparent additionally by a preservative coat of SAICOS Wood-Impregnation 0011 Pine transparent 9003* (let dry for at least 18 hrs). 0018 Sand transparent *Use biocides with caution. -

Wood Finishing Demonstration Project Final Report
Wood Finishing Demonstration Project Final Report Paul Pagel Minnesota Technical Assistance Program & Barb Loida Minnesota Pollution Control Agency Small Business Compliance Assistance Program January 1997 Table of Contents INTRODUCTION......................................................................................................................................... 1 FINDING AND SELECTING A CANDIDATE FOR THE PROJECT................................................... 1 THE WOOD FINISHING PROCESS......................................................................................................... 2 PROCESS CONSIDERATIONS AND COMPANY COMPARISONS............................................................................. 2 EMISSIONS AND WASTES ....................................................................................................................... 4 AT PINE-TIQUE ................................................................................................................................................ 4 AT VIKING ....................................................................................................................................................... 5 USE OF WATERBORNE FINISHES......................................................................................................... 6 FINISH CRITERIA AND PROCESS CONSIDERATIONS FOR SELECTING ALTERNATIVE COATINGS ......................... 6 TESTING, MODIFICATIONS AND RESULTS...................................................................................... -
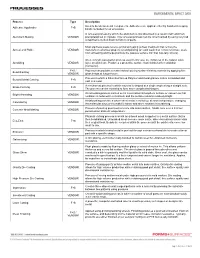
Process Type Description Adhesive Application F+S Used to Bond Non-Metal Components. Adhesives Are Applied Either by Hand Or In
Process Type Description Used to bond non-metal components. Adhesives are applied either by hand or in a spray Adhesive Application F+S booth controlled for air emissions. A two-step process by which the aluminum is first dissolved in a caustic bath and then Aluminum Making VENDOR precipitated out in crystals. This two-step process can be circumvented by using recycled scrap that is melted down to form new parts. Most stainless steels receive a final annealing (a heat treatment that refines the Anneal and Pickle VENDOR material's mechanical properties) and pickling (an acid wash that removes furnace scale from annealing and helps promote the passive surface film that naturally occurs). An electrolytic passivation process used to increase the thickness of the natural oxide Anodizing VENDOR layer on aluminum. Produces a protective surface that inhibits further oxidation (corrosion). F+S / Process that provides a matte/etched looking surface finish to material by applying fine Bead Blasting VENDOR glass beads at low pressure. Process in which a Fiber-Reinforced Polymer and metal granule matrix is blended and Bonded Metal Casting F+S cast in a mold. A mechanical process in which material is shaped at a single angle along a straight axis. Brake Forming F+S The process can be repeated to form more complicated shapes. An annealing process carried out in a controlled atmosphere furnace or vacuum so that Bright Annealing VENDOR oxidation is reduced to a minimum and the surface remains relatively bright. Metalworking process in which sheet metal is rolled out at room temperature, changing Calendaring VENDOR the molecular structure to make it harder and more resistant to scratching. -

Hirshfield's Paint Mfg., Inc
Hirshfield’s Paint Mfg., Inc. TECHNICAL DATA BULLETIN INTERIOR ACRYLIC WOOD STAIN 8410 Resin Type Sheen Quality Level Spread Rate Clean Up Thinner Dry at 77°F V.O.C. Level 300-500 sq. 6-10 min. tack professional 100% acrylic flat ft./gal. water water free 742 g/L best (on smooth surfaces) 1 hour topcoat Description Available Colors This unique interior water based acrylic stain provides all See interior wood stain card or display panels. the beauty of oil based stains without the harmful sol- Caution vents. It dries quickly to a luxurious, rich finish and leaves no harmful odor or residue. Keep from freezing. Where To Use See other cautions on back. Use this acrylic stain on almost every new wood surface Environmentally Friendly Tips or for re-do jobs. It is excellent, for use on interior doors, • Only purchase the quantity you need. millwork, trim and cabinets. Do not use on exterior sur- • Do not mix paints for storage or disposal. Leave paint faces. When in doubt, test stain in a hidden spot to be in the original container. sure the wood is properly prepared and compatible. • Do not throw liquid paint in the trash or pour it down Surface Preparation the drain • Small amounts of latex paint can be air-dried and dis- All surfaces to be stained must be clean and dry. Remove posed through your trash collection system. all wax, dust, oil, etc. Sand all new wood smooth. Previ- ous coatings must be completely removed. Use a water • Recycle leftover or unused paint. -

Instructions Pre-Stain Wood Conditioner Natural Oil Step 1
Instructions Pre-Stain Wood Conditioner Natural Oil Step 1: Preparation All raw wood projects require preparation sanding before applying stain or wood conditioners. If you skip this critical step, your finish may be rough and uneven. Preparation for Raw Wood Projects Prior to the Application of an Oil-based Stain See our video: How to Prep Sand Raw Wood 1. Prep sand with 120-grit sandpaper followed by 150-grit sandpaper. 2. Remove dust with a vacuum, compressed air, a tack cloth, or a water-dampened rag. 3. Do not over-sand with fine-grit sandpapers; this will close and seal the wood grain, preventing ideal product absorption. Preparation of An Existing Butcher Block Surface 1. Remove any food residue using a pastry scraper or spatula to gently scrape the countertop in areas where you find dried-on food or residue. Avoid gouging the wood by keeping the blade at an angle that just skims the surface. 2. Scrub the countertop using a scrub brush, Scotch Brite Pad or sponge and scrub the countertop with hot water and mild dish soap. 3. Rinse with hot water: Run a clean dishcloth through hot water and rinse the countertop several times. 4. Dry thoroughly with a clean dish towel or paper towel. How to Apply General Finishes Oil Based Pre-Stain Conditioner General Finishes Oil Based Pre-Stain Conditioner Application Steps For use with oil-based stains. 1. Stir General Finishes Oil Based Pre-Stain Conditioner to reincorporate solids that have settled to the bottom of the can. 2. Do NOT thin. -

Advanced Formula Oi L-Based Wood Stain R No. B3500 Series
TECHNICAL DATA SHEET ADVANCED FORMULA OI L-BASED WOOD STAIN R FPO NO. B3500 SERIES PRODUCT INFORMATION BEHR® Advanced Formula Oil-Based Wood Stain is a full-bodied penetrating, wiping & gel stain designed for easy application COMPLIES WITH THE BELOW AS OF 10/1/2019 to all interior wood surfaces such as cabinets, trim, doors, paneling, furniture and floors. This product provides one-coat SCAQMD YES OTC YES coverage and enhances the natural beauty of wood. CARB SCM 2007 YES LADCO YES RECOMMENDED USES: CARB YES AIM YES Ideal for properly prepared interior wood surfaces, both finished and unfinished, painted surfaces, fiberglass, coated metal and OTC PHASE II YES composition surfaces. Excellent for vertical and horizontal applications. PRODUCT SPECIFICATIONS: SURFACE PREPARATION: CAUTIONS/LIMITATIONS: Tint Base/Max Tint Load: All surfaces must be clean, free of dust, oil, grease, wax, • Always test stain on an inconsipicuous area of the wood to No. B3500 Tint Base 29 fl oz / 3 fl oz (quart) polish, glue and all other foreign substances. verify desired color. No. B3500 Tint Base 7.25 fl oz / 0.75 fl oz (half-pint) New Wood: Sand with #120-grit sandpaper followed by #180- • Shelf life under normal conditions is 2 years unopened. Premixed Colors: Available in 16 colors grit or finer for a smoother finish if desired. Remove all dust. • Contents are combustible. Keep away from heat and open Gloss: n/a Previously Finished Wood: Lightly sand surface with a 400- flame. grit sandpaper. Remove all dust. To ensure uniform stain color Resin Type: Oil and beautiful results on bare wood, apply BEHR Oil-Based Pre- Weight per Gallon: 6.6 lb Stain Wood Conditioner prior to staining. -

(12) United States Patent (10) Patent No.: US 6,737,155 B1 Ou (45) Date of Patent: May 18, 2004
USOO6737155B1 (12) United States Patent (10) Patent No.: US 6,737,155 B1 Ou (45) Date of Patent: May 18, 2004 (54) PAPER OVERLAID WOOD BOARD AND 4,492.726 A 1/1985 Rosenberg METHOD OF MAKING THE SAME 4,541,880 A 9/1985 Arena et al. 5,425,976 A 6/1995 Clarke et al. (76) Inventor: Nian-hua Ou, 1791 Julian Dr., E. A : 3.19. last s al. .......... 156/62.2 Watkinsville, GA (US) 30677 5,885,6852Y- - 2 A * 3/1999 Tingleyea e ......................a 428/105 (*) Notice: Subject to any disclaimer, the term of this E6; f : S. E, al... i.e., patent is extended or adjusted under 35 2- Y - - - - - - - - - - - - - - - - - - U.S.C. 154(b) by 373 days. * cited by examiner Primary Examiner-Cynthia H. Kelly (21) Appl. No.: 09/457,183 ASSistant Examiner-L. Ferguson (22) Filed: Dec. 8, 1999 (74) Attorney, Agent, or Firm-Carlos Nieves; David Mitchell Goodrich (51) Int. Cl." .............................. D21J 1/00; B32B 3/00; B32B5/12; B32B 29/02 (57) ABSTRACT (52) U.S. Cl. ................................ 1822,5: A paper Overlaid wood product having a relatively low s density. The board includes a core comprised of oriented (58) Field of Search ........................... 428/2924, 292.7, Strand board. A resin impregnated kraft paper Overlay is 428/105,137 adhesively Secured to the top Surface of the oriented Strand (56) References Cited board core. The paper Overlaid wood board has a density ranging from about 35 lb/ft to about 55 lb/ft, a modulus of U.S. PATENT DOCUMENTS rupture (MOR) of from about 3000 to about 9000 psi, a modulus of elasticity of about 0.4 million to about 1.2 3,308,013 A * 3/1967 Bryant ...................... -
Wood Finishing Basics
Wood Finishing Basics APPLICATION TECHNIQUES & PRODUCT SELECTION GETTING STARTED Your home is a unique expression of your individual Before starting any wood finishing project it is important decorating taste. And what better way to display your to have a proper workspace and supplies. When creating style than through the warmth and beauty of natural the ideal area please keep the following in mind: wood? From traditional to contemporary and everywhere • The work station must have adequate light. in-between, the possibilities are endless! Wood’s natural • Always work in a well ventilated area. warmth and beauty add richness and character. And with • The workspace should be dry and warm. If the area is ® a little know-how and Minwax stains and finishes, it’s cool or damp it may alter the dry times indicated on the easier than you think to create a statement of style. labels. In this guide, author and craftsman, Bruce Johnson, There are a number of items frequently used in wood pairs application tips and techniques with product finishing projects. This list does not include all the supplies needed, but what is most commonly used. recommendations that best enhance the beauty of wood Always consult the label of the Minwax® products you are in your home. This booklet will take you through the wood using to see if special application tools are required. finishing process including preparation, staining and topcoating, as well as maintenance of your wood pieces. Rags Stir Sticks Whether you are a beginner or a seasoned do-it-yourselfer, Brushes Safety Glasses these ideas will help you achieve beautiful results with ease.