Supporting Document 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
U.S. V. Bayer AG and Monsanto Company Comment: the Sierra Club
ATTN: Kathleen S. O'Neill Chief, Transportation, Energy & Agriculture Section Antitrust Division United States Department of Justice 450 5th Street, NW, Suite 800 Washington, DC 20530 Petition in opposition to proposed U.S. v. Bayer AG and Monsanto Company settlement and merger: A merger of agrochemical giants Bayer and Monsanto would create the world's largest seed and pesticide maker. I am afraid this move will reduce competition, raise prices for consumers and farmers, and result in an unacceptable degree of control over the agricultural industry and our food supply. I am very concerned about pollinators and the increased risks to bees, butterflies and birds with the increase of Bayer's neonicotinoids. Both companies produce corn products engineered to imply the use of harmful pesticides they manufacture. The production of corn uses high amounts of nitrogen- based fertilizers and the excess sediment is contaminating our waterways, therefore I am deeply worried about increased corn production from this merger. The heavy nutrient runoff from corn is widely attributed to exacerbating the marine "Dead Zone" in the Gulf of Mexico, in which algal blooms create hypoxic conditions wherein oxygen concentration is in such low levels that marine life suffocates and dies. I urge the Department of Justice to do more prevent the Bayer-Monsanto seed and pesticide platform from growing too strong by stopping this merger. If this merger is allowed, it should require more pesticide and seed divestments in order to protect our agriculture and food supply. This merger is anti-competition, if it is approved it will fail to protect farmers, consumers and the environment by allowing further consolidation of the industrial agriculture sector. -

Plant Viral, Agrobacterium Tumefaciens, and Xanthomonas Spp.
USDA May conta in Co nfidential Business Information ~ United States Department of Agricu lture . ... - ------------ --------- - - - ------------ Animal and August 28, 201 4 Plant Health Dr. Luc Mathus Inspection Service Cellectis Plant Sciences 600 County Road D West, Suite 8 Biotechnology Regulatory New Brighton, MN 55112 Servi ces 4700 I iver Road Re: Request for Confirmation that I. ] Potato is not a regulated article Unit 98 Riverdale, MD 20737 Dear Dr. Mathis: Thank you for your letter dated July 29, 2013 inquiring whether or not the potato product described in your letter is a regulated article. This letter states that the "potato has· improved consumer safety and processing attributes attributable to a single gene knock-out achieved through transient expression of a Transcription Activator-Like Effector Nuclease (TALEN)." APHIS regulates the introduction of certain genetically engineered organisms which are, or have the potential to be plant pest. Regulations for genetically engineered organisms that have the potenti al to be plant pests, under the Plant Protection Act, are codified at 7 CFR part 340, "Introduction of Organisms and Products Altered or Produced Through Genetic Engineering Which Are Plant Pest or Which There Is Reason To Believe Are Plant Pests." Under the provisions of these regulations, a genetically engineered (GE) organism is deemed a regulated article if it has been genetically engineered from a donor organism, recipient organism, or vector or vector agent listed in §340.2 and the listed organism meets the definition of "plant pest" or is an unclassified organism and/or an organism whose classification is unknown, or if the Administrator dete11nines that tl e GE organism is a plant pest or has reason to believe is a plant pest. -

The Era of Corporate Consolidation and the End of Competition Bayer-Monsanto, Dow-Dupont, and Chemchina-Syngenta
Research Brief October 2018 The Era of Corporate Consolidation and the End of Competition Bayer-Monsanto, Dow-DuPont, and ChemChina-Syngenta DISRUPT ECOSYSTEM ACCLERATE MONOPOLY THE EFFECTS OF CORPORATE CONSOLIDATION UNDERMINE FOOD SECURITY HARM SMALL PRODUCERS HAASINSTITUTE.BERKELEY.EDU This publication is published by the Haas Institute for a Fair and Inclusive Society at UC Berkeley This research brief is part of the Haas Institute's Shahidi Project from the Global Justice Program. The Shahidi Project (Shahidi is a Swahili word meaning “witness”) intends to demystify the power structures and capacities of transnational food and agricultural corporations within our food system. To that end, researchers have developed a robust database focusing on ten of the largest food and agricultural corporations in the world. See more at haasinstitute.berkeley.edu/shahidi. About the Authors Copyeditor Support Elsadig Elsheikh is the director Marc Abizeid Special thanks to the Food of the Global Justice program and Farm Communications at the Haas Institute for a Infographics Fund, which provided the seed Fair and Inclusive Society at Samir Gambhir funding for the Shahidi project. the University of California- Berkeley, where he oversees Report Citation Contact the program’s projects and Elsadig Elsheikh and Hossein 460 Stephens Hall research on corporate power, Ayazi. “The Era of Corporate Berkeley, CA 94720-2330 food system, forced migration, Consolidation and The End of Tel 510-642-3326 human rights, Islamophobia, Competition: Bayer-Monsanto, haasinstitute.berkeley.edu structural marginality and Dow-DuPont, and ChemChina- inclusion, and trade and Syngenta.” Haas Institute for development. a Fair and Inclusive Society at the University of California, Hossein Ayazi, PhD, is a Berkeley, CA. -
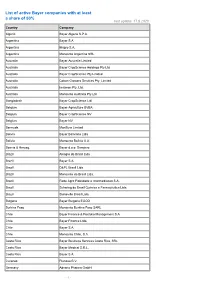
List of Active Bayer Companies with at Least a Share of 50% Last Update: 17.8.2020 Country Company
List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Algeria Bayer Algerie S.P.A. Argentina Bayer S.A. Argentina Biagro S.A. Argentina Monsanto Argentina SRL Australia Bayer Australia Limited Australia Bayer CropScience Holdings Pty Ltd Australia Bayer CropScience Pty Limited Australia Cotton Growers Services Pty. Limited Australia Imaxeon Pty. Ltd. Australia Monsanto Australia Pty Ltd Bangladesh Bayer CropScience Ltd. Belgium Bayer Agriculture BVBA Belgium Bayer CropScience NV Belgium Bayer NV Bermuda MonSure Limited Bolivia Bayer Boliviana Ltda Bolivia Monsanto Bolivia S.A. Bosnia & Herzeg. Bayer d.o.o. Sarajevo Brazil Alkagro do Brasil Ltda Brazil Bayer S.A. Brazil D&PL Brasil Ltda Brazil Monsanto do Brasil Ltda. Brazil Rede Agro Fidelidade e Intermediacao S.A. Brazil Schering do Brasil Química e Farmacêutica Ltda. Brazil Stoneville Brasil Ltda. Bulgaria Bayer Bulgaria EOOD Burkina Faso Monsanto Burkina Faso SARL Chile Bayer Finance & Portfolio Management S.A. Chile Bayer Finance Ltda. Chile Bayer S.A. Chile Monsanto Chile, S.A. Costa Rica Bayer Business Services Costa Rica, SRL Costa Rica Bayer Medical S.R.L. Costa Rica Bayer S.A. Curacao Pianosa B.V. Germany Adverio Pharma GmbH - 1 - List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Germany AgrEvo Verwaltungsgesellschaft mbH Germany Alcafleu Management GmbH & Co. KG Germany BGI Deutschland GmbH Germany Bayer 04 Immobilien GmbH Germany Bayer 04 Leverkusen Fußball GmbH Germany Bayer 04 Leverkusen -

Histochemical Analysis of Expression Pattern of Camv 35S Promoter in Transgenic Rapeseed (Brassica Napus L.)
Current World Environment Vol. 10(Special Issue 1), 752-757 (2015) Histochemical Analysis of Expression Pattern of CaMV 35S Promoter in Transgenic Rapeseed (Brassica napus L.) PARISSA JONOUBI1, ALI HateF SALMANIAN2 and AMIN RavaeI3 1Department of Plant Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran. 2Department of Plant Biotechnology, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran. 3Master of Science, Department of Plant Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran. http://dx.doi.org/10.12944/CWE.10.Special-Issue1.90 (Received: November, 2014; Accepted: April, 2015) ABstract Promoters are considered valuable tools for biotechnology and provide great opportunities for eugenic purposes. This study aimed to investigate the expression pattern of GUS gene directed by CaMV 35S promoter in transgenic rapeseed (Brassica napus L.). The GUS gene was transferred to the rapeseed explants by Agrobacterium. The regenerated and rooted transgenic plantlets were transferred to the pots containing a mixture of soli and vermiculite. These plants underwent PCR and histochemical GUS assay. The cross-section of leaf, petiole, and stem of the transformed plants was obtained. GUS activity was observed in phloem, parenchyma, collenchyma, and supporting tissue of vascular bundles. It was also observed in cambium, endoderm, vascular ray, pith parenchyma, epidermis, and trichomes. These results showed that CaMV 35S causes the expression of transgene in various tissues differently. -

Whither Plant Genetic Engineering? Allow Crops to Tolerate Environmental Stress Such As Drought, Cold, Salt, Heat, Or flood
PLANT TREK TO BOLDLY GO WHERE NO PLANT HAS GONE BEFORE On the Past, Present & Future of Plant Genetic Engineering by Richard G. Stout A HowPlantsWork.com eBook Copyright © 2013 by Richard G. Stout Version 1.0.1 PDF August, 2013 Table of Contents Preface Chapter 1: Where Do New Plants Come From? Chapter 2: How To Make A Transgenic Plant Chapter 3: Gene Guns, Terminators & Traitors Chapter 4: Farmaceuticals, Plantibodies & Edible Vaccines Chapter 5: Into The Wild Chapter 6: Are GM Plants Self-Replicating Inventions? Chapter 7: Plant Trek - The Next Generation Chapter 8: DIY Plant Genetic Engineering? Attributions About The Author Glossary about where plant biotechnology may be headed in the future, Preface including how plant biotechnology “hobbyists” may be getting into the act. Who is this book for? Please Note: This book is NOT a comprehensive textbook on plant genetic engineering and biotechnology. (If you’re looking This book is intended for people who may be curious about for such books, I’m sure you can find them at your local college plant genetic engineering, but who don’t want to read a long, bookstore or at an online bookseller.) Nor is this book meant to technical textbook on the subject. (There are provided, be a defense of genetically-engineered organisms (GMOs), however, ample links to books and articles - and also to online though I’m sure some readers will think so. Maybe here’s why. resources - for further reading.) If you’re looking for small “tastes” of information regarding various aspects of plant Since I was a graduate student in the 1970s at the University of genetic engineering, then this little book maybe just the Washington where some of the original work on transgenic informational “snack” that you’re looking for. -

The Ethics of Changing People Through Genetic Engineering, 13 Notre Dame J.L
Notre Dame Journal of Law, Ethics & Public Policy Volume 13 Article 5 Issue 1 Sym[posium on Emerging Issues in Technology 1-1-2012 What Sort of People Do We Want - The thicE s of Changing People through Genetic Engineering Michael J. Reiss Follow this and additional works at: http://scholarship.law.nd.edu/ndjlepp Recommended Citation Michael J. Reiss, What Sort of People Do We Want - The Ethics of Changing People through Genetic Engineering, 13 Notre Dame J.L. Ethics & Pub. Pol'y 63 (1999). Available at: http://scholarship.law.nd.edu/ndjlepp/vol13/iss1/5 This Article is brought to you for free and open access by the Notre Dame Journal of Law, Ethics & Public Policy at NDLScholarship. It has been accepted for inclusion in Notre Dame Journal of Law, Ethics & Public Policy by an authorized administrator of NDLScholarship. For more information, please contact [email protected]. WHAT SORT OF PEOPLE DO WE WANT? THE ETHICS OF CHANGING PEOPLE THROUGH GENETIC ENGINEERING MICHAEL J. REISS* Within the last decade, genetic engineering has changed from being a relatively esoteric research technique of molecular biologists to an application of considerable power, yet one which raises widespread public concern. In this article, I first briefly summarize the principles of genetic engineering, as applied to any organism. I then concentrate on humans, reviewing both progress to date and possible future developments. Throughout, my particular focus is on the ethical acceptability or otherwise of the technology. I restrict myself to cases where humans are themselves being genetically engineered. This means, for instance, that I do not cover such topics as xenotransplantation (when animals are genetically engineered to make them suitable for transplantation into humans) and the issues resulting from the production of such products as genetically engineered human growth hor- mone, al-antitrypsin, or vaccines (when micro-organisms, ani- mals, or plants are genetically engineered to produce human proteins). -

Import of DNA Into Mammalian Nuclei by Proteins Originating from a Plant Pathogenic Bacterium
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3729–3733, March 1999 Cell Biology Import of DNA into mammalian nuclei by proteins originating from a plant pathogenic bacterium i ALICJA ZIEMIENOWICZ*, DIRK GO¨RLICH†,ERICH LANKA§,BARBARA HOHN*¶, AND LUCA ROSSI* *Friedrich Miescher-Institut, P.O. Box 2543, CH-4002 Basel, Switzerland; †Zentrum fu¨r Molekulare Biologie der Universita¨tHeidelberg, Im Neuenheimer Feld 282, D-69120 Heidelberg, Germany; and §Max-Planck Institut fu¨r Molekulare Genetik, Ihnestrasse 73, D-14195 Berlin, Germany Communicated by Diter von Wettstein, Washington State University, Pullman, WA, February 2, 1999 (received for review October 22, 1998) ABSTRACT Import of DNA into mammalian nuclei is accompanying it into the plant. The bacterial proteins VirD2 generally inefficient. Therefore, one of the current challenges and VirE2 both contain bipartite nuclear localization signals in human gene therapy is the development of efficient DNA (NLSs) that target them into plant nuclei (7–9). Furthermore, delivery systems. Here we tested whether bacterial proteins the C-terminal NLS of the VirD2 protein is required for could be used to target DNA to mammalian cells. Agrobacte- efficient transfer of the bacterial T-DNA to the plant nucleus rium tumefaciens, a plant pathogen, efficiently transfers DNA (10–12). Because mammalian NLSs have been shown to be as a nucleoprotein complex to plant cells. Agrobacterium- recognized in plant systems (13–16), these target sequences mediated T-DNA transfer to plant cells is the only known may be universal. example for interkingdom DNA transfer and is widely used for We reconstituted in vitro the T-DNA complexes composed plant transformation. -

Agribusiness and Antitrust: the Bayer-Monsanto Merger, Its Legality, and Its Effect on the United States and European Union
The Global Business Law Review Volume 7 Issue 1 Article 9 7-1-2018 Agribusiness and Antitrust: The Bayer-Monsanto Merger, Its Legality, and Its Effect on the United States and European Union Aleah Douglas Cleveland-Marshall College of Law Follow this and additional works at: https://engagedscholarship.csuohio.edu/gblr Part of the Antitrust and Trade Regulation Commons, and the Business Organizations Law Commons How does access to this work benefit ou?y Let us know! Recommended Citation Aleah Douglas, Agribusiness and Antitrust: The Bayer-Monsanto Merger, Its Legality, and Its Effect on the United States and European Union, 7 Global Bus. L. Rev. 156 (2018) available at https://engagedscholarship.csuohio.edu/gblr/vol7/iss1/9 This Note is brought to you for free and open access by the Journals at EngagedScholarship@CSU. It has been accepted for inclusion in The Global Business Law Review by an authorized editor of EngagedScholarship@CSU. For more information, please contact [email protected]. AGRIBUSINESS AND ANTITRUST: THE BAYER-MONSANTO MERGER, ITS LEGALITY, AND ITS EFFECT ON THE UNITED STATES AND EUROPEAN UNION ALEAH DOUGLAS I. INTRODUCTION……………………………………………………………………... 157 II. BACKGROUND…………………………………………………………………….....158 A. A Review of the Bayer-Monsanto Merger………………………….…………... 158 B. United States Antitrust Laws…………………………………………………… 161 1. The Applicable Laws……………………………………………………. 161 2. Problems and Antitrust Violations……………………………………… 166 3. American Agribusiness…………………………………………………. 167 C. European Union Antitrust Laws……………………………………………….. 172 1. The European Union……………………………………………………. 172 2. The Applicable Laws………………………………….………………… 172 3. Problems and Antitrust Violations……………………….………....…... 174 4. European Union Agribusiness…………….…………………………….. 176 D. Illegality and Detriment …………………………………….…………………. 178 III. CONCLUSION……………………………………………………………………....... 180 ABSTRACT This note examines the current and historical antitrust laws of the United States and the European Union as they relate to the currently pending merger between Bayer and Monsanto. -

This Document Is the Property of Bayer AG And/Or
Safety Assessment Summary of Genuity Roundup Ready 2Yield MON 89788 Soybean Executive Summary Ongoing developments in biotechnology and molecular-assisted breeding have enabled Monsanto to develop a second-generation glyphosate-tolerant soybean product: GenuityTM Roundup Ready 2 Yield® or MON 89788 soybean (OECD Unique ID: MON–89788–1). Similar to the first generation product Roundup Ready® soybean, MON 89788 soybean will continue to provide growers with flexibility, simplicity, and cost effective weed control options. However, MON 89788 soybean and varieties containing the trait have the added potential toand and regime. enhance yield and thereby further benefit farmersAG and the soybean industry. In 1996, Roundup Ready soybean was the first soybean product containing a biotechnology trait to be commercialized in the U.S. Roundup Ready soybean was produced by incorporation of the cp4 epspsBayer coding property sequence derivedpublishing from the protectioncontents common soil bacterium Agrobacteriumof sp. strain CP4. The cp4 epsps coding its sequence directs the production of the 5-enolpyruvylparties. datashikimate -3-phosphatetherefore and/oror synthase (termed CP4 EPSPS) that is much less sensitive to glyphosate inhibition affiliates. than endogenous plant EPSPS.property The CP4 EPSPSintellectualthird renders Roundup Readymay soybean its tolerant to glyphosate, which is the asactive ingredient in Roundup agricultural the of and herbicides. The utilizationis of Roundup agriculturalregulatory herbicides plusowner. Round up a document -

Barley Transformation Using Agrobacterium-Mediated Techniques
Barley Transformation using Agrobacterium-Mediated Techniques Wendy A Harwood, Joanne G Bartlett, Silvia C Alves, Matthew Perry, Mark A Smedley, Nicola Leyland and John W Snape Abstract Methods for the transformation of barley using Agrobacterium-mediated techniques have been available for the past ten years. Agrobacterium offers a number of advantages over Biolistic-mediated techniques in terms of efficiency and the quality of the transformed plants produced. This chapter describes a simple system for the transformation of barley based on the infection of immature embryos with Agrobacterium tumefaciens followed by the selection of transgenic tissue on media containing the antibiotic hygromycin. The method can lead to the production of large numbers of fertile, independent transgenic lines. It is therefore ideal for studies of gene function in a cereal crop system. Key Words: Barley transformation, Agrobacterium tumefaciens, transgenic plants, hygromycin, immature embryo. 1 1. Introduction The first reports of successful barley transformation (1) used biolistic-based techniques to introduce DNA to immature embryos. Immature embryos were also the target tissue used in the first reports of the generation of transgenic barley plants using Agrobacterium (2). Although alternative target tissues have been examined for use in barley transformation systems, immature embryos remain the target tissue of choice for obtaining high transformation efficiencies. An alternative Agrobacterium- mediated barley transformation system uses microspore cultures as the target tissue (3). A comparison of biolistic and Agrobacterium-based methods for barley transformation highlighted some of the advantages of the Agrobacterium system (4). These included higher transformation efficiencies, lower transgene copy number and more stable inheritance of the transgenes with less transgene silencing. -

The Bio Revolution: Innovations Transforming and Our Societies, Economies, Lives
The Bio Revolution: Innovations transforming economies, societies, and our lives economies, societies, our and transforming Innovations Revolution: Bio The The Bio Revolution Innovations transforming economies, societies, and our lives May 2020 McKinsey Global Institute Since its founding in 1990, the McKinsey Global Institute (MGI) has sought to develop a deeper understanding of the evolving global economy. As the business and economics research arm of McKinsey & Company, MGI aims to help leaders in the commercial, public, and social sectors understand trends and forces shaping the global economy. MGI research combines the disciplines of economics and management, employing the analytical tools of economics with the insights of business leaders. Our “micro-to-macro” methodology examines microeconomic industry trends to better understand the broad macroeconomic forces affecting business strategy and public policy. MGI’s in-depth reports have covered more than 20 countries and 30 industries. Current research focuses on six themes: productivity and growth, natural resources, labor markets, the evolution of global financial markets, the economic impact of technology and innovation, and urbanization. Recent reports have assessed the digital economy, the impact of AI and automation on employment, physical climate risk, income inequal ity, the productivity puzzle, the economic benefits of tackling gender inequality, a new era of global competition, Chinese innovation, and digital and financial globalization. MGI is led by three McKinsey & Company senior partners: co-chairs James Manyika and Sven Smit, and director Jonathan Woetzel. Michael Chui, Susan Lund, Anu Madgavkar, Jan Mischke, Sree Ramaswamy, Jaana Remes, Jeongmin Seong, and Tilman Tacke are MGI partners, and Mekala Krishnan is an MGI senior fellow.