Display PDF in Separate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

80B Torpoint - Seaton - Liskeard
80B Torpoint - Seaton - Liskeard A Line Travel Timetable Valid from 14/01/2013 Until Further Notice Direction of stops: where shown (eg: W-bound) this is the compass direction towards which the bus is pointing when it stops Mondays to Fridays Service Restrictions Sch SH Torpoint, Torpoint Ferry (SW-bound) 0655 1007 1220 1510 1528 1810 Torpoint, Carbeile Inn (W-bound) 1008 1222 1522 1530 1812 Torpoint, School (NW-bound) 0658 1530 Torpoint, opp Torpoint Bus Depot 1531 Torpoint, HMS Raleigh (W-bound) 0700 1012 1225 1533 1533 1815 Antony, Ring O' Bells (W-bound) 0703 1015 1228 1536 1536 1818 Sheviock, Opposite Sheviock Church (NW-bound) 0706 1018 1231 1539 1539 1821 Polbathic, West Park (W-bound) 0709 Crafthole, opp Cross Park 1021 1234 1542 1542 1824 Portwrinkle, Finnygook Beach (E-bound) 1024 1237 1545 1545 1827 Downderry, opp Church 1037 1250 1558 1558 1838 Seaton, opp The Car Park 1040 1253 1601 1601 1841 Hessenford, Opposite the Old Mill (W-bound) 1046 1259 1607 1607 1847 Widegates, Antiques Shop (W-bound) 1050 1302 1610 1610 1850 Liskeard, Charter Way Morrisons (NE-bound) 1100 1312 1620 1620 Liskeard, Hospital (S-bound) 1103 1315 1623 1623 Liskeard, Post Office (S-bound) 0735 1106 1318 1626 1626 1900 Liskeard, opp Railway Station 1110 1321 1629 1629 1903 Saturdays Torpoint, Torpoint Ferry (SW-bound) 0655 1007 1220 1528 1810 Torpoint, Carbeile Inn (W-bound) 1008 1222 1530 1812 Torpoint, School (NW-bound) 0658 Torpoint, opp Torpoint Bus Depot Torpoint, HMS Raleigh (W-bound) 0700 1012 1225 1533 1815 Antony, Ring O' Bells (W-bound) 0703 -

MPC Agenda 18 Jan 2018
12 January 2018 Dear Councillor You are summoned to a meeting of the Council which will be held on Thursday 18 January 2018 at 7.30 p.m. at Menheniot Old School when your presence is requested. The business to be transacted is shown on the Agenda below. Please note that the general public are invited to attend this and any meeting of The Parish Council. This meeting is advertised as a public meeting and as such could be filmed or recorded by broadcasters, the media and members of the public. Please be aware that whilst every effort is taken to ensure that members of the public will not be filmed, we cannot guarantee this, especially if you are speaking or taking an active role. Yours faithfully John Hesketh Clerk to the Council Minute Agenda Items 1/2018 Chairman’s Welcome 2/2018 Apologies for Absence 3/2018 Members’ Declarations A. Pecuniary/Registerable Declarations of Interests – Members must declare an interest, which has been declared on their Register of Financial Interests Form, relevant to the agenda. B. Non-registerable Interests – Members must declare non-pecuniary interests at the start of the meeting or whenever the interests become apparent. C. Declaration of Gifts – Members are reminded they must declare any gift or hospitality with a value in excess of fifty pounds. D. Dispensations – Members to consider any written requests for dispensations. 4/2018 Public Participation Members of the public are invited to address the Council. (15 minutes) Report from Cornwall Councillor Phil Seeva 5/2018 Minutes of Meeting Councillors will be asked to AGREE to accept the following minutes as a true and accurate record. -

THE WIVES of the Lees of QUETHIOCK & THEIR ANCESTRY
THE WIVES OF THE LEEs of QUETHIOCK & THEIR ANCESTRY Let us now make a helpmate for this man, A creature like himself, Then he made Eve. It is evident from this, proof positive, That woman is for man`s help and delight, His comfort and his earthly paradise, How can they but live in unity? One flesh they are; and one flesh, so I`d guess, Has but one heart, come grief or happiness. So if you are man not a mouse Thank God for sending you a wife; Geoffrey Chaucer -Canterbury Tales 1386 The ancestors of the wives of the ancestral Lee`s of Quethiock, that I have identified, bore 73 different family names. Of these families, 53 came from the corner of southeast Cornwall that lies within 20 miles of Plymouth; 13 came from elsewhere in Cornwall and 7 from outside the county. All those from outside the county are the ancestors of my mother, Florence Maud Clarke. All of those of my father were from Cornwall But in addition to the above 73 names we know another 23 probable ones. These are the family names of the ancestors of Jane Coode, who married William Lee (1535?-1607) and had a daughter Jane. This Jane I believe, but cannot prove conclusively, is the Jane Lee who is recorded in the Parish Records of Quethiock Church as marrying in 1581 my 8th greatgrandfather John, the nephew of William Lee. If so, then the ancestors of Jane Coade are mine too. The Coades were armigerous gentry and so their family trees were recorded by the Heralds during their Visitations to Cornwall in 1530,1573 & 1620 and are listed by J.L.Vivien in his publication of 1887, `Heraldic Visitations of Cornwall`, of which I was able to purchase an original copy from the Antique Booksellers in Bristol. -

Padstow Go Cornwall Bus
Plymouth Citybus Plymouth - Bodmin Parkway Go Cornwall11 Bus via Saltash - Landrake - Tideford - Liskeard - Dobwalls Bodmin Parkway - Padstow Go Cornwall11A Bus via Bodmin - Wadebridge Callywith College Days Ref.No.: 17K1 Service No 11 11A 11 11A 11A 11 11A 11 11A 11 11A 11 11A 11 11A 11 11A 11 11A 11A 11 11A 11 11A 11 11A ROYAL PARADE 0720 0840 0940 1040 1140 1240 1340 1440 1540 1640 1740 Railway Station, Saltash Road 0724 0844 0944 1044 1144 1244 1344 1444 1544 1644 1744 Milehouse, Alma Road 0727 0847 0947 1047 1147 1247 1347 1447 1547 1647 1747 St Budeaux, Square 0736 0856 0956 1056 1156 1256 1356 1456 1556 1656 1756 Saltash, Fore Street 0741 0901 1001 1101 1201 1301 1401 1501 1601 1701 1801 Broad Walk, Saltash School 1505 Cornwall College 1507 Callington Road, shops 0744 0904 1004 1104 1204 1304 1404 1508 1604 1704 1804 Burraton, Plough Green 0747 0907 1007 1107 1207 1307 1407 1511 1607 1707 1807 Landrake, footbridge 0752 0912 1012 1112 1212 1312 1412 1516 1612 1712 1812 Tideford, Quay Road 0755 0915 1015 1115 1215 1315 1415 1519 1615 1715 1815 Trerulefoot, Garage 0759 0919 1019 1119 1219 1319 1419 1523 1619 1719 1819 Lower Clicker, Hayloft 0802 0922 1022 1122 1222 1322 1422 1526 1622 1722 1822 Liskeard, Charter Way (Morrisons) 0807 0927 1027 1127 1227 1327 1427 1531 1627 1727 1827 Liskeard, Dental Centre 0810 0930 1030 1130 1230 1330 1430 1534 1630 1730 1830 Liskeard, Post Office 0815 0935 1035 1135 1235 1335 1435 1539 1635 1735 1835 Liskeard, Post Office 0740 0820 0940 1040 1140 1240 1340 1440 1540 1640 1740 1840 Dobwalls, Methodist -

Barrowmore Model Railway Journal·
ISSN 1745-9842 Barrowmore Model Railway Journal· Number33 December 2012 Published on behalf ofBarrowmore Model Railway Group by the Honorary Editor: David Goodwin, "Cromef', Church Road, Saughall, Chester CHI 6EN; tel. 01244 880018. E-mail: [email protected] Contributions are welcome: (a) as e-mails or e-mail attachments; (b) a hard copy of a computer file; (c) a typed manuscript; (d) a hand-written manuscript, preferably with a contact telephone number so that any queries can be sorted out; (e) aCD/DVD; (f) a USB storage flash drive. · Any queries to the Editor, please. The NEXT ISSUE will be dated March 2013, and contributions should get to the Editor as soon as possible, but at least before 1 February 2013. ++111I11l++H+++l1 I 111 1111111II111++++11I111111++++++++1III11II11111111 1111++++1I11111 Copies of this magazine are also available to non-members: a cheque for £9 (payable to 'Barrowmore Model Railway Group') will provide the next four issues, posted direct to your home. Send your details and cheque to the Editor at the above address. I I I I 11III11 11IIII11 I l++I 111 I I I I I I 11 I I I 11III11IIIIl+II11 I 11 I l++++++++++++++++++++I I I I I I I I The cover illustration for this issue is one of a couple of photographs of Barrowmore, of unknown provenance, discovered by Harry Wilson when he was unpacking some belongings transferred from his former house in Tarvin. Readers will recall that Harry rented a couple of units at Barrowmore, using them for storage of the book-stock of his bookselling business. -

Penzance to Mousehole Penzance to Lands
A1 Penzance to Lands End via Sennen Mondays to Saturdays except bank holidays towards Lands End towards Penzance Penzance Bus Station [B] 0635 1740 Lands End Car Park 0729 1836 Green Market 0638 1743 Sennen opp FIrst & Last 0734 1841 Mounts Bay School Sennen Cove 0739 1846 St Clare Crows-an-Wra Bus Shelter 0747 1854 Humphry Davy School 1510 St Buryan opp Post Office 0752 1859 12 Newlyn Coombe 1520 Lamorna Turn 0759 1906 Newlyn Bridge 1522 1748 Sheffield South Place 0804 1911 Gwavas Crossroads 1525 1751 Paul Boslandew Hill 0807 1914 Paul Boslandew Hill 1527 1753 Gwavas Crossroads 0810 1917 Sheffield Belgravia Place 1530 1756 Newlyn Bridge 0813 1920 Lamorna Turn 1535 1801 Newlyn Coombe 0815 Drift opp Elstree 0649 Mounts Bay Academy St Buryan Post Office 0655 1542 1808 opp Humphry Davy School Due to COVID 19 restrictions these trips are Crows-an-Wra opp bus shelter 1547 Market Jew Street 0822 1929 School services only & closed to the public Sennen Cove 1554 Penzance Bus Station [B] 0828 1935 Treen Bus Shelter 0703 1815 Porthcurno Car Park 0709 1821 Lands End Car Park 0724 1836 Additional Journeys on this route are run by First Kernow please see their timetables for more information. M6 Penzance to Mousehole via Newlyn Mondays to FridaysSaturdays School except days bank only holidays Mounts Bay Academy 1455 Treneere Stores 1500 Humphry Davy School 1510 Alverton The Ropewalk 1516 Newlyn Bridge The Strand 1520 Newlyn opp Red Lion 1523 Mousehole The Old Coastguard Hotel 0755 1528 13 Newlyn Red Lion 0800 Newlyn Bridge 0803 Alverton The Ropewalk -

11 Plymouth to Bodmin Parkway Via Dobwalls | Liskeard | Tideford | Landrake | Saltash
11 Plymouth to Bodmin Parkway via Dobwalls | Liskeard | Tideford | Landrake | Saltash COVID 19 Mondays to Saturdays Route 11 towards Bodmin Route 11 towards Plymouth Plymouth Royal Parade (A7) 0835 1035 1235 1435 1635 1835 1935 Bodmin Parkway Station 1010 1210 1410 1610 1810 2010 Railway Station Saltash Road 0839 1039 1239 1439 1639 1839 1939 Trago Mills 1020 1220 1420 1620 Milehouse Alma Road 0842 1042 1242 1442 1642 1842 1942 Dobwalls Methodist Church 1027 1227 1427 1627 1823 2023 St Budeaux Square [S1] 0850 1050 1250 1450 1650 1849 1949 Liskeard Lloyds Bank 0740 0840 1040 1240 1440 1640 1840 2032 Saltash Fore Street 0855 1055 1255 1455 1655 1854 1954 Liskeard Dental Centre 0741 0841 1041 1241 1441 1641 1841 Callington Road shops 0858 1058 1258 1458 1658 1857 1957 Liskeard Charter Way Morrisons 0744 0844 1044 1244 1444 1644 1844 Burraton Plough Green 0900 1100 1300 1500 1700 1859 1959 Lower Clicker Hayloft 0748 0848 1048 1248 1448 1648 1848 Landrake footbridge 0905 1105 1305 1505 1705 1904 2004 Trerulefoot Garage 0751 0851 1051 1251 1451 1651 1851 Tideford Quay Road 0908 1108 1308 1508 1708 1907 2007 Tideford Brick Shelter 0754 0854 1054 1254 1454 1654 1854 Trerulefoot Garage 0911 1111 1311 1511 1712 1910 2010 Landrake footbridge 0757 0857 1057 1257 1457 1657 1857 Lower Clicker Hayloft 0914 1114 1314 1514 1715 1913 2013 Burraton Ploughboy 0802 0902 1102 1302 1502 1702 1902 Liskeard Charter Way Morrisons 0919 1119 1319 1519 1720 1918 2018 Callington Road shops 0804 0904 1104 1304 1504 1704 1904 Liskeard Dental Centre 0921 1121 1321 1521 -

Map 3 Lower Clicker
Tendering & Procurement for Grass Cutting, Weed Spraying & Strimming Invitation to quote and information for applicants Basic contract information Menheniot Parish Council is inviting contractors, individuals and voluntary groups to tender for a three year contract to cut grass, spray weeds and strim within the parish boundary. Procurement of goods and services by the parish council is only necessary when the contract value is higher than £60,000. However, the council is committed to working in an open, transparent and accountable way, and is distributing these documents across the public domain. For this reason, we are inviting applications in order to give everyone the widest opportunity to tender for the work. Section 1 explains what declarations you need to make and what you need to tell us. Section 2 sets out a timetable for the tendering process: a final decision will be taken by parish councillors in a closed session at their public meeting on 16 February 2017. The council is allowed to meet in closed session where there is commercially confidential business to be transacted (Public Bodies (Admission to Meetings) Act 1960 s1.2). Section 3 explains how we will decide who to award the contract to. Contract title Grass cutting, weed spraying and strimming in Menheniot parish Contract length 3 years from 1 April 2017 with annual performance reviews. Basic description of services The key requirements of this contract are: Grass cutting the sports field on East Road Strimming of verges around the parish Weed spraying around the parish Local Maintenance Partnership (small works contract from Cornwall Council) Documents Maps and plans of the areas covered by this agreement are attached in the Appendices, together with a list of what work is required from each. -
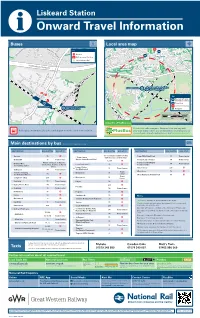
Liskeard Station I Onward Travel Information Buses Local Area Map
Liskeard Station i Onward Travel Information Buses Local area map TB Key M A Bus Stop P Town Centre Rail replacement Bus Stop Station Entrance/Exit A38 Liskeard by-pass 1 0 m i n u t A38 e s w a l k i n g d i s t a n c e Liskeard Branch Station e e c c Liskeard Station n n a a t t (Looe Valley Line) s s i i d d g g n B n i i k k l A l a a w w Key s s e e t t u u M Museum & Tourist n n i i Information Centre m m 0 0 1 1 P Thorn Park TB Town Centre Bus Stop Liskeard Station Liskeard Branch Station (Looe Valley Line) Footpaths km 0 0.5 0 Miles 0.25 Liskeard is a area. PlusBus Contains Ordnance Survey data © Crown copyright and database right 2018 & also map data © OpenStreetMap contributors, CC BY-SA PlusBus is a discount price ‘bus pass’ that you buy with Rail replacement buses/coaches will depart from the front of the station your train ticket. It gives you unlimited bus travel around your chosen town, on participating buses. Visit www.plusbus.info Main destinations by bus (Data correct at September 2019) DESTINATION BUS ROUTES BUS STOP DESTINATION BUS ROUTES BUS STOP DESTINATION BUS ROUTES BUS STOP Antony 75 B 10 - 15 minutes walk from this Trago Mills Retail Park 11 Town Centre - Town Centre station (see Local area map) Bodmin 11 Town Centre (Barras Street/Post Office) Tremar/Lower Tremar 74 Town Centre 75, 236 B Please see buses to Darite/ Tremarcoombe/Higher Bodmin Moor 74 Town Centre Crows Nest, St Cleer or Upton Looe/East Looe ^ 73 A Tremarcoombe (for walking trails) Cross. -

Tavistock World Heritage Site Key Centre Steering Group Interpretation Strategy
Tavistock World Heritage Site Key Centre Steering Group Interpretation Strategy Andrew Thompson January 2014 Tavistock Town Centre © Barry Gamble Contents Introduction p2 1. Statement of Significance p7 2. Interpretation Audit p13 3. Audience Research p22 4. Interpretive Themes p29 5. Standards for Interpretation p44 6. Recommendations p47 7. Action Plan p59 Appendix: Tavistock Statement of Significance p62 Bibliography p76 Acknowledgement The author is grateful to Alex Mettler and Barry Gamble for their assistance in preparing this strategy. 1 Introduction This strategy sets out a framework and action plan for improving interpretation in Tavistock and for enabling the town to fulfil the requirements of a Key Centre within the Cornwall and West Devon Mining Landscape World Heritage Site (WHS). It is intended to complement the Tavistock World Heritage Site Key Centre Learning Strategy (Kell 2013) which concentrates on learning activities and people. Consequently the focus here is primarily on interpretive content and infrastructure rather than personnel. Aims and objectives The brief set by the Tavistock World Heritage Site Key Centre Steering Group was to identify a consistent, integrated approach to presenting the full range of themes arising from the Outstanding Universal Value of WHS Areas 8, 9 and 10 and to respond to the specific recommendations arising from the WHS Interpretation Strategy (WHS 2005). We were asked to: x Address interpretation priorities in the context of the Cornish Mining WHS x Identify and prioritise target audiences x Set out a clearly articulated framework and action plan for the development of interpretation provision in WHS Area 10, including recommendations which address x Product development (i.e. -

Gilliflower Orchard (Formerly Lostwithiel Golf Club) Cott Road Lostwithiel PL22 0HQ
Suveys ltd Suveys Ltd Surveys Ecological Gilliflower Orchard (formerly Lostwithiel Golf Club) Cott Road Lostwithiel PL22 0HQ GR: SX 11242 60643 November 2020 xxxxxx ASR_Gilliflower_Smit_November_2020 Contents 1 Delivery of Tree Related Information into the Planning System. ............................................ 3 2 Summary of Content ............................................................................................................. 4 3 Site Details ............................................................................................................................ 6 4 Development Proposal .......................................................................................................... 7 5 Tree Data Schedule of Results.............................................................................................. 8 6 Site Images ......................................................................................................................... 12 7 Arboricultural Impact Assessment (AIA) .............................................................................. 13 8 Development Proposal Assessment. ................................................................................... 14 a. Wildlife and Ecological Perspective: - Bat Roost/Bird Nest .......................................... 14 b. How the Development Proposal Affects Local Character - ........................................... 15 c. Shadow Influence on Dwellings/Buildings/Amenity Space. .......................................... 15 d. -

Edited by IJ Bennallick & DA Pearman
BOTANICAL CORNWALL 2010 No. 14 Edited by I.J. Bennallick & D.A. Pearman BOTANICAL CORNWALL No. 14 Edited by I.J.Bennallick & D.A.Pearman ISSN 1364 - 4335 © I.J. Bennallick & D.A. Pearman 2010 No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior permission of the copyright holder. Published by - the Environmental Records Centre for Cornwall & the Isles of Scilly (ERCCIS) based at the- Cornwall Wildlife Trust Five Acres, Allet, Truro, Cornwall, TR4 9DJ Tel: (01872) 273939 Fax: (01872) 225476 Website: www.erccis.co.uk and www.cornwallwildlifetrust.org.uk Cover photo: Perennial Centaury Centaurium scilloides at Gwennap Head, 2010. © I J Bennallick 2 Contents Introduction - I. J. Bennallick & D. A. Pearman 4 A new dandelion - Taraxacum ronae - and its distribution in Cornwall - L. J. Margetts 5 Recording in Cornwall 2006 to 2009 – C. N. French 9 Fitch‟s Illustrations of the British Flora – C. N. French 15 Important Plant Areas – C. N. French 17 The decline of Illecebrum verticillatum – D. A. Pearman 22 Bryological Field Meetings 2006 – 2007 – N. de Sausmarez 29 Centaurium scilloides, Juncus subnodulosus and Phegopteris connectilis rediscovered in Cornwall after many years – I. J. Bennallick 36 Plant records for Cornwall up to September 2009 – I. J. Bennallick 43 Plant records and update from the Isles of Scilly 2006 – 2009 – R. E. Parslow 93 3 Introduction We can only apologise for the very long gestation of this number. There is so much going on in the Cornwall botanical world – a New Red Data Book, an imminent Fern Atlas, plans for a new Flora and a Rare Plant Register, plus masses of fieldwork, most notably for Natural England for rare plants on SSSIs, that somehow this publication has kept on being put back as other more urgent tasks vie for precedence.