CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sacred Water Bodies of Kumaradhara River and Natural
Lake 2016: Conference on Conservation and Sustainable Management of Ecologically Sensitive Regions in Western Ghats [THE 10TH BIENNIAL LAKE CONFERENCE] th Date: 28-30 December 2016, http://ces.iisc.ernet.in/energy Venue: V.S. Acharya Auditorium, Alva's Education Foundation, Sundari Ananda Alva Campus, Vidyagiri, Moodbidri, D.K. Dist., Karnataka, India – 574227 SACRED WATER BODIES OF KUMARADHARA RIVER AND NATURAL PONDS IN DAKSHINA KANNADA DISTRICT, KARANATAKA Chaithanya Kedila* and Deviprasad K.N.** * III BSc Student, Vivekananda college Puttur D.K .Karnataka ** Associate Professor, Vivekananda college Puttur D.K .Karnataka [email protected] Abstract__ Natural ponds and sacred water bodies are bodies in Kumaradhara rivers have more an integral component of the hydrological system and significances in the point of conservation of rare perform diverse roles in the biosphere. Studies of species of fishes. Studies of these ecosystems are these ecosystems are often neglected, probably, due to often neglected, probably, due to lack of lack of knowledge about their significances. The knowledge about the significance of these objectives of this study is to create public awareness and to provide the basic information to the concerned ecosystems. Wetland conservation is an important authorities to restore the original condition of these program of ecological importance. Natural ponds, ecosystems. The study was carried out at different which are being neglected, should be given seasons in the year 2016. A survey of 12 natural importance. The objectives of this study are to ponds in Dakshina Kannada and five sacred water create public awareness and to provide the basic bodies of Kumaradhara river revealed that they are information to the concerned authorities to restore the important source of water and rare biodiversity. -

Impact Assessment of the Karnataka Litigation Policy and Karnataka Sakala Services Act 2011 in Reducing Government Litigation in the State of Karnataka
594 123286/2019/NM Impact Assessment of the Karnataka Litigation Policy and Karnataka Sakala Services Act 2011 in reducing Government Litigation in the State of Karnataka Ministry of Law and Justice Government of India National Law School of India University Bengaluru 2018 595 123286/2019/NM ACKNOWLEDGEMENT A meaningful research study involves the cooperation and support of many individuals. I take this small opportunity to express my gratitude to all the people and institutions without whose support the completion of this research study would have been a distant dream. At the outset I am grateful to the Department of Justice, Ministry of Law and Justice, Government of India for initiating the Scheme for Action Research and Studies on Judicial Reforms and providing NLSIU the opportunity to conduct the research study on “Impact Assessment of the Karnataka Litigation Policy and Karnataka Sakala Services Act 2011 in reducing Government Litigation in the State of Karnataka”. I am thankful to all the official members of the Department of Justice for providing all the necessary support in conducting this study. I express my deepest gratitude to the Honourable Judges of the Karnataka High Court and Subordinate Courts for providing all the data relating to delay and pendency in the conduct of government litigation. I am equally grateful to all the officials in the Department of Law and Justice, Government of Karnataka for interacting with the research team and highlighting the several challenges faced in the conduct of government litigation. I am thankful to all the government advocates who interacted with the research team and provided their candid opinion on the litigation policy. -

Agricultural Coolie, Residing at Kukkandoor Quarters, Jalsoor Village, Sullia, Dakshina Kannada-574 239
: 1 : IN THE HIGH COURT OF KARNATAKA AT BANGALORE DATED THIS THE 22 ND DAY OF JANUARY 2013 BEFORE THE HON’BLE MR.JUSTICE K.N.KESHAVANARAYANA CRL.P.No.6703/2012 C/w CRL.P.Nos.6671/2012 AND 6726/2012 In Crl.P.No.6703/2012: BETWEEN : S.Mahaveer S/o. Subramanya, Aged about 37 years, Occ: Agricultural Coolie, Residing at Kukkandoor Quarters, Jalsoor Village, Sullia, Dakshina Kannada-574 239. ... Petitioner (Now in Judicial Custody) [By Sri.R.B.Deshpande, Advocate] AND : The State of Karnataka, By CID Police, Bangalore for Sullia Police Station-574 239. … Respondent [By Sri.Rajesh Rai.K., Government Pleader] This Criminal Petition is filed under Section 439 of the Cr.P.C. praying to enlarge the petitioner on bail in Crime No.53/2008 of Sullia Police Station, Dakshina Kannada, for the offences punishable under Sections 448, 323 and 302 read with Section 34 of IPC. : 2 : In Crl.P.No.6671/2012: BETWEEN : S.Shivaraj S/o. Subramani, Aged about 37 years, Occ: Agricultural Coolie, C/o C-Coop Ivarnadu, Ivarnadu Village, Sullia, Dakshina Kannada-574 239. ... Petitioner (Now in Judicial Custody) [By Sri.R.B.Deshpande, Advocate] AND : The State of Karnataka, By CID Police, Bangalore for Sullia Police Station-574 239. … Respondent [By Sri.Rajesh Rai.K., Government Pleader] This Criminal Petition is filed under Section 439 of the Cr.P.C. praying to enlarge the petitioner on bail in Crime No.53/2008 of Sullia Police Station, Dakshina Kannada, for the offences punishable under Sections 448, 323 and 302 read with Section 34 of IPC and charge sheet for the offences punishable under Sections 448, 396, 397 and 120-B of IPC. -

Relationship of Oral Hygiene Practices and Dental Caries
Global Journal of Medical research Dentistry and Otolaryngology Volume 13 Issue 2 Version 1.0 Year 2013 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4618 & Print ISSN : 0975-5888 Relationship of Oral Hygiene Practices and Dental Caries among School Children of Sullia Taluk, Karnataka, South India By Praveena S, Thippeswamy HM, Nanditha K & Kalyana Chakravarthy P Manipal University, India Abstract-Objective: we aimed to evaluate the prevalence of dental caries, treatment needs and oral hygiene practices school going children of Sullia taluk. Materials and methods: A total of 1800 school children constituted the study sample. Each age group consisted of 600 children. Information on oral hygiene methods was collected. Dental caries was recorded using dft/DMFT as per WHO 1997 guidelines. Results: The prevalence of dental caries was found to be 33.6% in Sulliataluk. The prevalence of dental caries was found among 5 year old 31.0%, 12 year old 32.8% and 15 year old 37.0% respectively. Prevalence of dental caries among tooth brush using 32.6% and finger users 42.8%. This observation was statistically significant (P<0.05). The percentage of caries affected children was low among tooth paste user (30.5%) and those who brush their teeth twice daily (10.6%). Keywords: dental caries, treatment need. GJMR-J Classification : NLMC Code: WU 158 RelationshipofOralHygienePracticesandDentalCariesamongSchoolChildrenofSulliatalukKarnatakaSouthIndia Strictly as per the compliance and regulations of: © 2013. Praveena S, Thippeswamy HM, Nanditha K & Kalyana Chakravarthy P. This is a research/review paper, distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License http://creativecommons.org/licenses/by- nc/3.0/), permitting all non-commercial use, distribution, and reproduction inany medium, provided the original work is properly cited. -

SLR Residency
SLR Residency https://www.indiamart.com/slr-residency/ Kukke Subramanya is one of the most wonderful and devotional places in the state of Karnataka. It is situated in a place surrounded by the hills of the western ghats. Lord Subramanya is the principal deity worshiped. About Us Kukke Subramanya is one of the most wonderful and devotional places in the state of Karnataka. It is situated in a place surrounded by the hills of the western ghats. Lord Subramanya is the principal deity worshiped in this temple in the form of a Cobra. Hotel SLR Residency is a comfortable hotel, ideally located in the heart of Kukke Subramanya, 5 minutes from the KSRTC Bus Stand . Guests may choose rooms with or without air-conditioning. Each well appointed guest room has a private bathroom, television. The State of Karnataka has always been tourist destination on account of its Rich Natural Beauty. Dakshina Kannada is always attarction for Tourists for its varied Heritage and also the Presence of several Centuries. The Dakshina Kannada has an abundance of Natural resources, Beautiful beaches,forests and mountains, Coconut plantations and Rivers.There are famous age-old Temples in and around Dakshina Kannada which have from time Immemorial been considered as holy places of pilgrimage. generally, people coming to the City would surely make it a point to visit these temples. To Name a few Kukke Subramanya Temple, Kateel Shree Durga Parameshawri Temple, Mangaladevi Temple, Kadri Ishwar Temple, Dharmasthala Manjunatha Temple, Kollur Mookambika Devi Temple are some of such famous Temples. People from all over India/world are been visiting these Temples. -

CRLP4977-14-12-01-2016.Pdf
- 1 - IN THE HIGH COURT OF KARNATAKA AT BENGALURU DATED THIS THE 12 TH DAY OF JANUARY 2016 BEFORE THE HON’BLE MRS.JUSTICE RATHNAKALA CRIMINAL PETITION NO.4977/2014 BETWEEN: 1. IBRAHIM GOONADKA S/O HASSAINAR HAJI AGED ABOUT 57 YEARS MEMBER OF ADVISORY COMMITTEE GOONADKA BADRIYA MASJID R/O GOONADKA HOUSE SAMPAJE VILLAGE, SULLIA TALUK DAKSHINA KANNADA – 574 234 2. KUMBAKOD MAHAMMAD S/O KUMBAKOD MOIDU KUNHI AGED ABOUT 55 YEARS R/O GOONADKA HOUSE SAMPAJE VILLAGE, SULLIA TALUK DAKSHINA KANNADA – 574 234 3. KHADAR KUMBAKOD S/O KUMBAKOD MOIDU KUNHI AGED ABOUT 30 YEARS R/O GOONADKA HOUSE SAMPAJE VILLAGE, SULLIA TALUK DAKSHINA KANNADA – 574 234 4. MUSTAFA BALNAD S/O BALNAD IBRAHIM AGED ABOUT 45 YEARS R/O DHARKASTU, GOONADKA SAMPAJE VILLAGE, SULLIA TALUK DAKSHINA KANNADA – 574 234 - 2 - 5. SMT.HAJIRA W/O MOHAMMED KUNHI, AGED ABOUT 40 YEARS R/O GOONADKA HOUSE SAMPAJE VILLAGE, SULLIA TALUK DAKSHINA KANNADA – 574 234 6. HARIS S/O SOOFI AGED ABOUT 40 YEARS R/O DHARKASTU, GOONADKA SAMPAJE VILLAGE, SULLIA TALUK DAKSHINA KANNADA – 574 234 …PETITIONERS (BY SRI SIDDHARTH B. MUCHANDI, ADV.) AND: 1. THE STATE OF KARNATAKA BY THE POLICE OF SULLIA POLICE STATION DAKSHINA KANNADA – 574 239 2. T.M.SHAHID S/O T.M.BABA AGED ABOUT 40 YEARS PRESIDENT PERADKA MOYYADDIN JUMMA MASJID R/O THEKKIL HOUSE SULLIA TALUK DAKSHINA KANNADA – 574 239. ...RESPONDENTS (BY SRI CHETAN DESAI, HCGP FOR R1; SRI M.R.BALAKRISHNA, ADV. FOR R2) THIS CRIMINAL PETITION IS FILED UNDER SECTION 482 OF CR.P.C., PRAYING TO QUASH THE ENTIRE PROCEEDINGS AGAINST THEM IN C.C.NO.16/2014 (PCR NO.38/12) FOR THE OFFENCE P/U/S 506 R/W 34 OF IPC AND SECTION 66 (A) (b) OF THE IT ACT, ON THE FILE OF THE C.J. -
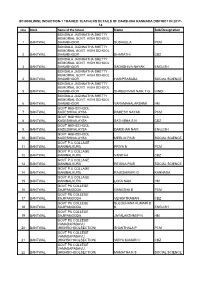
List of Trained Teachers Induction-1 Dakshina Kannada
SCHOOLWISE INDUCTION-1 TRAINED TEACHERS DETAILS OF DAKSHINA KANNADA DISTRICT IN 2017- 18 slno Block Name of the School Name Sub/Designation BONDALA JAGNNATHA SHETTY MEMORIAL GOVT. HIGH SCHOOL 1 BANTWAL SHAMBHOOR SUSHEELA PCM BONDALA JAGNNATHA SHETTY MEMORIAL GOVT. HIGH SCHOOL 2 BANTWAL SHAMBHOOR BHARATHI CBZ BONDALA JAGNNATHA SHETTY MEMORIAL GOVT. HIGH SCHOOL 3 BANTWAL SHAMBHOOR SADASHIVA NAYAK ENGLISH BONDALA JAGNNATHA SHETTY MEMORIAL GOVT. HIGH SCHOOL 4 BANTWAL SHAMBHOOR HARIPRASADA SOCIAL SCIENCE BONDALA JAGNNATHA SHETTY MEMORIAL GOVT. HIGH SCHOOL 5 BANTWAL SHAMBHOOR SHREENIVAS NAIK T G HINDI BONDALA JAGNNATHA SHETTY MEMORIAL GOVT. HIGH SCHOOL 6 BANTWAL SHAMBHOOR VARAMAHALAKSHMI HM GOVT HiGHSCHOOL 7 BANTWAL KADESHIVALAYSA RAMESH NAYAK PCM GOVT HiGHSCHOOL 8 BANTWAL KADESHIVALAYSA SATHISHA S N CBZ GOVT HiGHSCHOOL 9 BANTWAL KADESHIVALAYSA DARSHAN NAIK ENGLISH GOVT HiGHSCHOOL 10 BANTWAL KADESHIVALAYSA MERLIN PAIS SOCIAL SCIENCE GOVT P.U COLLAGE 11 BANTWAL MANINALKURU PRIYA N PCM GOVT P.U COLLAGE 12 BANTWAL MANINALKURU VANITHA CBZ GOVT P.U COLLAGE 13 BANTWAL MANINALKURU REGINA PAIS SOCIAL SCIENCE GOVT P.U COLLAGE 14 BANTWAL MANINALKURU RAJESHWARI G KANNADA GOVT P.U COLLAGE 15 BANTWAL MANINALKURU LOKA NAIK HM GOVT PU COLLEGE 16 BANTWAL SAJIPAMOODA GANESHA B PCM GOVT PU COLLEGE 17 BANTWAL SAJIPAMOODA VENKATRAMAN CBZ GOVT PU COLLEGE SULOCHANA KUMARI B 18 BANTWAL SAJIPAMOODA K ENGLISH GOVT PU COLLEGE 19 BANTWAL SAJIPAMOODA JAYALAKSHMI P N HM GOVT PU COLLEGE VAMADAPADAVU 20 BANTWAL (HIGHSCHOOLSECTION) SHANTHALA P PCM GOVT PU COLLEGE VAMADAPADAVU -

Mangalore Electricity Supply Company Limited
Mangalore Electricity Supply Company Limited Scheduled Outage Information Details of Power Shut Down due to maintenance of Distribution System from 27.06.2021 to 03.07.2021 Division: MANGALORE-1 RAPDRP & NON-RAPDRP FROM TO APPROXIMATE DIVISION SUBDIVISION SUBSTATION FEEDER_NAME SECTION DURATION OF AREA EFFECTED REASON FOR POWER OUTAGE DATE TIME DATE TIME POWER OUTAGE NIL Division: KAVOOR RAPDRP FROM TO APPROXIMATE DIVISION SUBDIVISION SUBSTATION FEEDER_NAME SECTION DURATION OF AREA EFFECTED REASON FOR POWER OUTAGE DATE TIME DATE TIME POWER OUTAGE Kavoor Kavoor Kavoor Kottara Kuloor 29.06.2021 9:30 29.06.2021 16:30 7:00 kottara junction Maintenance Work Kavoor Kavoor 220Kv Srs Kavoor Mullakadu Kavoor 30.06.2021 9:30 30.06.2021 16:30 7:00 A.J Hospital, Mullakadu Maintenance Work Kavoor Kavoor 220Kv Srs Kavoor Derebail Kavoor 30.06.2021 9:30 30.06.2021 16:30 7:00 Derebail Konchady, Kuntikana, Prashanthanagara, Maintenance Work Kavoor Kavoor 220Kv Srs Kavoor Malemar Kavoor 30.06.2021 9:30 30.06.2021 16:30 7:00 malemar, bolpugudde Maintenance Work Kavoor Surathkal 33Kv/11Kv Katipalla Surathkal Suratkal 01.07.2021 10:00 01.07.2021 15:00 5:00 Suratkal city Maintenance Work NITK, Srinivas nagar, mukka, udayanagara, bhandaramane, Kavoor Surathkal 33Kv/11Kv Katipalla Kudcemp Ug Suratkal 01.07.2021 10:00 01.07.2021 15:00 5:00 Maintenance Work konkanabail Kavoor Kavoor 220Kv Srs Kavoor Pachanady Kavoor 01.07.2021 9:30 01.07.2021 16:30 7:00 bondel junction, pachanady, krishna nagara, achukodi, vamanjur Maintenance Work Division: KAVOOR NON-RAPDRP -

Tourism Resources and Tourist Visitation in Selected Tourist Places
Tourism Resources and Tourist Visitation in Selected Tourist Places of Dakshina Kannada District, Karnataka – A Study Sheker Naik Department of Tourism and Travel Management, Mangalore University, Mangalagangothri Abstract Tourism is an important socio-economic and cultural activity. Today tourism resources are identified and developed with necessary tourist infrastructures throughout the world. Currently India is ranked 34th in the world out of 141 economies considered for the study by World Economic Forum in its Travel and Tourism Competitive Index Report of 2019. Tourism is gaining momentum in Karnataka, the southern state of India and the same is true in the case of Dakshina Kannada district as per as tourism resources and tourists arrivals are concerned. This study presents the digitisation important tourist attractions of the district besides making an analysis of tourist statistics during five years from 2012 to 2016. The study finds that the district has immense potential for tourism development and a lot needs to be done in order to attract the attention of more tourists to the district. Keywords: ArcGIS, Beach, Geo-reference, Tourism, Tourist. 1. Introduction Dakshina Kannada (DK) is a district in the southwestern part of coastal Karnataka. The district is sandwiched between the biological hotspot of Western Ghats in the east and the Arabian Sea in the west. The district enjoys great diversity in its physical and cultural settings. People of the districts are friendly, hospitable and honest. District has beautiful places of tourists‟ interest like temples, Basadis churches, mosques, beaches, Parks, peaks and many cultural and heritage attractions. Being in the strategic location, DK is bestowed with premier education centres and universities popularly known as educational hub of Karnataka as students from different parts of the country and abroad come here to study. -

Presence of Cheek Dimples and Absence of Palmaris Longus - an Anatomical Correlation from Dakshina Kannada, Karnataka
Jebmh.com Original Research Article Presence of Cheek Dimples and Absence of Palmaris Longus - An Anatomical Correlation from Dakshina Kannada, Karnataka Praveen Mulky Shenoy1, Amith Ramos2, Bharath Shetty3, Aravind Pallipady4 1Department of Anatomy, Srinivas Institute of Medical Sciences, Mangalore, Karnataka, India. 2Department of Anatomy, K.S. Hegde Medical Academy, Mangalore, Karnataka, India. 3Department of Forensic Medicine, K.V.G. Medical College and Hospital, Sullia, Dakshina Kannada, Karnataka, India. 4Department of Pathology, A.J. Institute of Medical Sciences and Research Centre, Mangalore, Karnataka, India. ABSTRACT BACKGROUND Palmaris longus (PL) tendon agenesis varies from 5 % to 30 % in different ethnic Corresponding Author: groups worldwide. Its agenesis is associated with decreased wrist grip, pinch grip, Dr. Amith Ramos, Associate Professor, presence of cheek dimples, difference of prevalence of agenesis with gender and Department of Anatomy, handedness and with flexor carpi superficialis tendon. Student and general K.S. Hegde Medical Academy, population surveys done in previous studies shows the association between these Mangalore, Karnataka, India. variables. The purpose of this study was to find out the association between the E-mail: [email protected] presence of cheek dimples with absence of palmaris long tendon. DOI: 10.18410/jebmh/2021/474 METHODS We examined 1200 medical and allied health students (600 males, 600 females) How to Cite This Article: Shenoy PM, Ramos A, Shetty B, et al. aged 18 - 24 years to assess the incidence of palmaris longus absence and the Presence of cheek dimples and absence presence of cheek and chin dimples. The presence or absence of palmaris longus of palmaris longus - an anatomical was assessed by clinical inspection using standard tests. -

Research Article
Rajeshwari N. et al / Int. J. Res. Ayurveda Pharm. 11 (1), 2020 Research Article www.ijrap.net (ISSN:2229–3566) LICHEN GENUS USNEA IN KARNATAKA Rajeshwari N. 1, Archana Ramachandra Mesta 2*, Vinayaka K S 3 1 Associate Professor, Department of Botany, Sahyadari Science College, Shimoga, Karnataka, India 2 Research Scholar, Department of Botany, Kumadvathi First Grade College, Shimoga Road, Shikaripura, Shimoga, Karnataka, India 3 Associate Professor, Department of Botany, Kumadvathi First Grade College, Shimoga Road, Shikaripura, Shimoga, Karnataka, India Received on: 18/09/19Accepted on: 04/11/19 *Corresponding author E-mail: [email protected] DOI: 10.7897/2277-4343.110114 ABSTRACT Usnea is a well-known genus of lichen for its medicinal uses all over the world. The lichen genus Usnea has fruticose morphology with pendulous or erect thallus and characterized by the presence of usnic acid. The present study aims to know the diversity of Usnea in Karnataka. The genus Usnea is identified up to species level based on its morphological, anatomical and chemical characters. The different forests types in Karnataka were surveyed for the work. We recorded 6 species of Usnea from the different parts of Karnataka. The higher altitudinal regions of the temperate forests harbours rich source of Usnea. Keywords: Karnataka, Usnea, Usnic acid, fruticose thallus INTRODUCTION parts of Karnataka, such as Shimoga, Chikkamagaluru, Uttara Kannada, Dakshina Kannada, Udupi, Dharwad, Belgaum, Gadag, The state Karnataka is situated in the south western part of India. Haveri, Ballary, Chitradurga, Davanagere, Tumkuru, The geography of Karnataka contains all types of topographical Chikaballapura, Kolara, Mysore, Chamarajnagar and Kodagu variations such as mountains, coastal areas, hills and plateaus. -

KVG Ayurveda Medical College & Hospital, Sullia Course
Name of the College: KVG Ayurveda Medical College & Hospital, Sullia Course: Under Graduation (BAMS) Academic Year: 2016-2017 Sl. Name of the Student along Father Name / Permanent Date of Blood Seat DOB Sex Mother’s Name Caste Contact No. No with photograph Husband’s name Address Admission Group Category Peringattuthodiyil House Mr. Fijin Backer Mohamed Firos P Sajidha C. Irimbiliyam Post 1. 01/02/1998 M GM 9497580072 13/10/2016 B+ P Tirur TQ Malappuram Dist KERALA. Management Seats Management Nampozhil House 1st mile Amaravathi Mr. Deepak Ben Kumily Post 15/11/1997 M Benny Bindu GM 9947928989 13/10/2016 O+ 2. Varghese Perumedu TQ, Idukki Dist KERALA , Seats Management Pin : 685509 Vadakkettu House 3. Shaiby James Kodumpidy Mr. Dael James 07/03/1998 M James Mathew GM 8547321101 13/10/201 B+ Kottayam Dist KERALA Pin : 686651 Seats Management Muttarkulangara House Anikkam Vellora Post 4. Sreelekha K. Mr. Anuraj Rajasekharan Kannur Dist 05/03/1998 M K. GM 9744105238 14/10/2016 O+ M.R. M.K. KERALA Pin: 670306 Seats Management Varada Thalappoyil Kariyathankave Ms.Anatha Post 5. 10/07/1998 F Rajakumaran T.P. Sheeja M. K. GM 9400544540 14/10/2016 A+ Meenu T.R. Thamarassery TQ Kozhikode Dist KERALA Pin: 673612 Seats Management Cheruvathur Kariyil Post Kasaragod Dist D/o. Shreenivasan Reena V P KERALA Pin: Ms. Shilpa V.P. 04/10/1998 F GM 9747978167 15/10/2016 B+ 6. V.V. 671313 Seats Management Babulmihraj House Perode Post Vadakara 7. Ms. Fida Jamal 27/02/1998 Sareena F Jamal A.K.