North American Buteos with This Charac- Seen by Many Birders but Did Not Return the Next Year
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Around the Region Are Published for Interest Only; Their Inclusion Does Not Imply Acceptance by the Records Committee of the Relevant Country
Sandgrouse31-090402:Sandgrouse 4/2/2009 11:24 AM Page 91 AROUND THE R EGION Dawn Balmer & David Murdoch (compilers) Records in Around the Region are published for interest only; their inclusion does not imply acceptance by the records committee of the relevant country. All records refer to 2008 unless stated otherwise. Records and photographs for Sandgrouse 31 (2) should be sent by 15 June to [email protected] ARMENIA Plover Dromas ardeola at Maharraq on 13 Aug Breeding bird surveys in 2008 in extreme NW was the first for several years. The 2nd record Armenia, near the border with Turkey and of Indian Roller Coracias benghalensis for Georgia (lake Arpilich and adjacent areas, Bahrain was at Badaan farm 5 Oct–15 Nov; the Shirak province), produced 43 new species first record was in Aug 1996 at Dair. The 3rd recorded for the area. Of these, 27 were record of Green Bee- eater Merops orientalis proven to breed there, including Egyptian was at Badaan farm on 29 Nov; the first since Vulture Neophron percnopterus, Booted Eagle several were recorded on the Hawar islands in Aquila pennata, Corncrake Crex crex, Eurasian 2000. Three Dark- throated Thrushes Turdus Eagle Owl Bubo bubo and Barred Warbler atrogularis were found on 20 Dec at Duraiz Sylvia nisoria. Significant breeding range and the 7th record of Chaffinch Fringilla extension for the country also noted here for coelebs was a female that was trapped and Little Bittern Ixobrychus minutus, Blue Rock ringed at Badaan farm on 29 Nov. A House Thrush Monticola solitarius and Meadow Pipit Bunting Emberiza striolata at Badaan farm on Anthus pratensis. -

A Multi-Gene Phylogeny of Aquiline Eagles (Aves: Accipitriformes) Reveals Extensive Paraphyly at the Genus Level
Available online at www.sciencedirect.com MOLECULAR SCIENCE•NCE /W\/Q^DIRI DIRECT® PHYLOGENETICS AND EVOLUTION ELSEVIER Molecular Phylogenetics and Evolution 35 (2005) 147-164 www.elsevier.com/locate/ympev A multi-gene phylogeny of aquiline eagles (Aves: Accipitriformes) reveals extensive paraphyly at the genus level Andreas J. Helbig'^*, Annett Kocum'^, Ingrid Seibold^, Michael J. Braun^ '^ Institute of Zoology, University of Greifswald, Vogelwarte Hiddensee, D-18565 Kloster, Germany Department of Zoology, National Museum of Natural History, Smithsonian Institution, 4210 Silver Hill Rd., Suitland, MD 20746, USA Received 19 March 2004; revised 21 September 2004 Available online 24 December 2004 Abstract The phylogeny of the tribe Aquilini (eagles with fully feathered tarsi) was investigated using 4.2 kb of DNA sequence of one mito- chondrial (cyt b) and three nuclear loci (RAG-1 coding region, LDH intron 3, and adenylate-kinase intron 5). Phylogenetic signal was highly congruent and complementary between mtDNA and nuclear genes. In addition to single-nucleotide variation, shared deletions in nuclear introns supported one basal and two peripheral clades within the Aquilini. Monophyly of the Aquilini relative to other birds of prey was confirmed. However, all polytypic genera within the tribe, Spizaetus, Aquila, Hieraaetus, turned out to be non-monophyletic. Old World Spizaetus and Stephanoaetus together appear to be the sister group of the rest of the Aquilini. Spiza- stur melanoleucus and Oroaetus isidori axe nested among the New World Spizaetus species and should be merged with that genus. The Old World 'Spizaetus' species should be assigned to the genus Nisaetus (Hodgson, 1836). The sister species of the two spotted eagles (Aquila clanga and Aquila pomarina) is the African Long-crested Eagle (Lophaetus occipitalis). -

Reproduction and Behaviour of the Long-Legged Buzzard (.Buteo Rufinus) in North-Eastern Greece
© Deutschen Ornithologen-Gesellschaft und Partner; download www.do-g.de; www.zobodat.at Die Vogelwarte 39, 1998: 176-182 Reproduction and behaviour of the Long-legged Buzzard (.Buteo rufinus) in North-eastern Greece By Haralambos Alivizatos, Vassilis Goutner and Michael G. Karandinos Abstract: Alivizatos , H., V. Goutner & M. G. Karandinos (1998): Reproduction and behaviour of the Long- legged Buzzard ( Buteo rufinus) in North-eastern Greece. Vogelwarte 39: 176-182. The breeding biology of the Long-legged Buzzard ( Buteo rufinus) was studied in the Evros area, north-eastern Greece in 1989, 1990, 1992 and 1993. The mean number of young fledged per pair per year was similar between years with an overall average of 0.93 (1.58 per successful pair). Of ten home range variables examined, the num ber of alternative nest sites and the extent of forest free areas in home ranges were significant predictors of nest ling productivity. Aggressive interactions were observed with 18 bird species (of which 12 were raptors), most commonly with the Buzzard {Buteo buteo). Such interactions declined during the course of the season. Prey pro visioning to nestlings was greatest in the morning and late in the afternoon declining in the intermediate period. Key words: Buteo rufinus, reproduction, behaviour, Greece. Addresses: Zaliki 4, GR-115 24 Athens, Greece (H. A.); Department of Zoology, Aristotelian University of Thessaloniki, GR-54006, Thessaloniki, Macedonia, Greece (V. G.); Laboratory of Ecology and Environmental Sciences, Agricultural University of Athens 75 Iera Odos 1 1855 Athens, Greece (M. G. K.). 1. Introduction The Long-legged Buzzard (Buteo rufinus) is a little known raptor of Europe. -

Effect of Environmental Elements on Migration Pattern of Eagles at Jorbeer Conservation Reserve, Bikaner, Rajasthan, India
Kataria et al RJLBPCS 2016 www.rjlbpcs.com Life Science Informatics Publications Original Research Article DOI - 10.26479/2016.0203.08 EFFECT OF ENVIRONMENTAL ELEMENTS ON MIGRATION PATTERN OF EAGLES AT JORBEER CONSERVATION RESERVE, BIKANER, RAJASTHAN, INDIA A.K. Kataria1*, N.Kataria2 and R.N.Kumawat3 1.Principal Investigator, Centre for excellence for use of space based technology in animal science, Rajasthan University of Veterinary and Animal Sciences, Bikaner-334001, Rajasthan, India. 2.Professor & Head, Department of Veterinary Physiology, College of Veterinary and Animal Science, Rajasthan University of Veterinary and Animal Sciences, Bikaner-334001, Rajasthan, India. 3. Deputy Forest Officer (Wildlife), Bikaner, Rajasthan ABSTRACT: The present endeavor was carried out to find out the effect of environmental elements on migration pattern of eagles at Jorbeer Conservation Reserve, Bikaner, Rajasthan, India (JCRBRI) during period from April 2015 to July 2016. The eagles studied were steppe eagle (Aquila nipalensis) and greater spotted eagle (Clanga clanga). The greater spotted eagle was seen only from November to February reiterating their winter migration to this reserve. Steppe eagle stayed at reserve during various months of the study year 2015 and 2016 when the THI values varied as 56.64-79.5. Recording of count of steppe eagle and greater spotted eagle besides real-time observation of environmental temperature and humidity signified in monitoring of eagle residence in the region. Result of the endeavor assisted in comprehending the absence of eagles in summer months during study periods. Residential period of steppe eagle was from November to April and of greater spotted eagle was from November to February only. -

Gori River Basin Substate BSAP
A BIODIVERSITY LOG AND STRATEGY INPUT DOCUMENT FOR THE GORI RIVER BASIN WESTERN HIMALAYA ECOREGION DISTRICT PITHORAGARH, UTTARANCHAL A SUB-STATE PROCESS UNDER THE NATIONAL BIODIVERSITY STRATEGY AND ACTION PLAN INDIA BY FOUNDATION FOR ECOLOGICAL SECURITY MUNSIARI, DISTRICT PITHORAGARH, UTTARANCHAL 2003 SUBMITTED TO THE MINISTRY OF ENVIRONMENT AND FORESTS GOVERNMENT OF INDIA NEW DELHI CONTENTS FOREWORD ............................................................................................................ 4 The authoring institution. ........................................................................................................... 4 The scope. .................................................................................................................................. 5 A DESCRIPTION OF THE AREA ............................................................................... 9 The landscape............................................................................................................................. 9 The People ............................................................................................................................... 10 THE BIODIVERSITY OF THE GORI RIVER BASIN. ................................................ 15 A brief description of the biodiversity values. ......................................................................... 15 Habitat and community representation in flora. .......................................................................... 15 Species richness and life-form -

Extreme Variation in the Tails of Adult Harlan’S Hawks
EXTREME VARIATION IN THE TAILS OF ADULT HARLAN’S HAWKS William S. (Bill) Clark Some adult Harlan’s Hawks have tails somewhat similar to this one Bob Dittrick But many others have very different tails, both in color and in markings Harlan’s Hawk type specimen. Audubon collected this adult in 1830 in Louisiana (USA) and described it as Harlan’s Buzzard or Black Warrier - Buteo harlani It is a dark morph, the common morph for this taxon. British Museum of Natural History, Tring Harlan’s Hawk Range They breed in Alaska, Yukon, & ne British Columbia & winter over much of North America. It occurs in two color morphs, dark and light. The AOS considers Harlan’s Hawk a subspecies of Red- tailed Hawk, Buteo jamaicensis harlani, but my paper in Zootaxa advocates it as a species. Clark (2018) Taxonomic status of Harlan’s Hawk Buteo jamaicensis harlani (Aves: Accipitriformes) Zootaxa concludes: “It [Harlan’s Hawk] should be considered a full species based on lack of justification for considering it a subspecies, and the many differences between it and B. jamaicensis, which are greater than differences between any two subspecies of diurnal raptor.” Harlan’s Hawk is a species: 1. Lack of taxonomic justification for inclusion with Buteo jamaicensis. 2. Differs from Buteo jamaicensis by: * Frequency of color morphs; * Adult plumages by color morph, especially in tail pattern and color; * Neotony: Harlan’s adult & juvenile body plumages are almost alike; whereas those of Red-tails differ. * Extent of bare area on the tarsus. * Some behaviors. TYPE SPECIMEN - Upper tail is medium gray, with a hint of rufous and some speckling, wavy banding on one feather, & wide irregular subterminal band. -

The Use of Green Plant Material in Bird Nests to Avoid Ectoparasites
July1984] ShortCommunications 615 the Spot-wingedFalconet may not benefitthe Monk HoY, G. 1980. Notas nidobio16gicasdel noroestear- Parakeet in such a manner. gentino. II. Physis (Buenos Aires), Secc. C, 39 We are grateful to A. G6mez Dur&n and J. C. Vera (96): 63-66. (INTA) for grantingus the useof the fieldwork areas, MACLEAN,G.L. 1973. The SociableWeaver, part 4: to N. Arguello and M. Nores for their assistancein predators, parasites and symbionts. Ostrich 44: the identification of food remains, and to J. Navarro 241-253. for his help in the field. This work wassupported by STRANECK,R., & G. VASINA. 1982. Unusual behav- a grant from the Subsecretariade Ciencia y Tecno- iour of the Spot-winged Falconet (Spiziapteryx logla (SUBCYT) of Argentina. circumcinctus).Raptor Res. 16: 25-26. LITERATURE CITED Received7 July 1983, accepted21 February1984. DEAN, A. 1971. Notes on Spiziapteryxcircumcinctus. Ibis 113: 101-102. The Use of Green Plant Material in Bird Nests to Avoid Ectoparasites PETER H. WIMBERGER 1 ZoologyDivision, Washington State Museum DB-10, Universityof Washington, Seattle,Washington 98105 USA Certain birds characteristicallyplace green plant causesnestling mortality in and nest desertion by material in their nests.This greenery is not part of birds (Webster 1944, Neff 1945, Fitch et al. 1946, Moss the nest structureproper but is placed haphazardly and Camin 1970, Feare 1976,Wheelwright and Boers- around the edges or inside the nest. The birds re- ma 1979).In general,the increasedmortality due to plenishthe spraysof greenmaterial, often daily, dur- ectoparasitesis causedby the loss of blood, which ing incubation and the nestling period (Brown and weakens the host, by viral disease, or by disease Amadon 1968, Beebe1976, pers. -

Migration Strategies of Common Buzzard (Buteo Buteo Linnaeus
Travaux du Muséum National d’Histoire Naturelle «Grigore Antipa» Vol. 60 (2) pp. 537–545 DOI: 10.1515/travmu-2017-0008 Research Paper Migration Strategies of Common Buzzard (Buteo buteo Linnaeus, 1758) in Dobruja Cătălin-Răzvan STANCIU1, Răzvan ZAHARIA2, Gabriel-Bogdan CHIȘAMERA4, Ioana COBZARU3, *, Viorel-Dumitru GAVRIL3, 1, Dumitru MURARIU3 1Faculty of Biology, University of Bucharest, 91–95 Splaiul Independenței, 5050095 Bucharest, Romania 2Oceanographic Research and Marine Environment Protection Society Oceanic-Club, Constanța, Romania 3Institute of Biology Bucharest of Romanian Academy, 296 Splaiul Independenței, 060031 Bucharest, Romania 4“Grigore Antipa” National Museum of Natural History, 1 Kiseleff Blvd., 011341, Bucharest, Romania *corresponding author, email: [email protected] Received: August 2, 2017; Accepted: August 31, 2017; Available online: August 31, 2017; Printed: December 31, 2017 Abstract. We studied various aspects regarding migration behavior of the Common Buzzard for two subspecies (B. b. buteo and B. b. vulpinus) transiting the region which overlaps with the Western Black Sea Corridor. Using vantage points set across Dobruja we managed to count 2,662 individuals. We highlighted the seasonal and diurnal peak passage, flight directions and height of flight for each season. Our results suggest that 57% of the counted individuals belongs to long-distance migrant Steppe Buzzard - B. b. vulpinus. The peek passage period in autumn migration was reached between the 26th of September to the 6th of October, while for the spring migration peek passage remained uncertain. The main autumn passage direction was from N to S, and NNW to SSE but also from NE to SW. For spring passage the main direction was from S to N but also from ESE to WNW. -

Family Break Up, Departure, and Autumn Migration in Europe of A
462 SHORT COMMUNICATIONS VOL. 39, NO. 4 j RaptorRes. 39(4):462-466 ¸ 2005 The Raptor ResearchFoundation, Inc. FAMILY BREAKUP, DEPARTURE,AND AUTUMN MIGRATION IN EUROPE OF A FAMILY OF GREATERSPOTTED EAGLES (AQUILA CIANGA) AS REPORTEDBY SATELLITETELEMETRY BERND-U. MEYBURG 1 WorldWorking Group on Birdsof Prey,Wangenheimstr. 32, D-14193 Berlin, Germany CHRISTIANE MEYBURG WorldWorking Group on Birdsof Prey,31 Avenuedu Maine, F-75015 Paris,France TADEUSZ MIZERA AND GRZEGORZ M•CIOROWSKI AgriculturalUniversity, Zoology Department, Wojska Polskiego 71C, 60-625Poznan, Poland JAN KOWALSKI Gugny2, 19-104 Trzcianne,Poland KEYWORDS: GreaterSpotted Eagle; Aquila clanga; depar- Terminals, PTTs) in the Biebrza river valleyof northeast- ture;family breakup; migration;satellite telemetry. ern Poland. The eagle nestwas located in a National Park protecting the largest peatlands in Central Europe, in- cluding 15547 ha of forests,18 182 ha of agricultural The transition of birds of prey to independenceis dif- land, and 25 494 ha of wetlands--the Biebrza marshes. ficult to study (Brown and Amadon 1968), as both old More than 70 natural and semi-naturalplant associations and young birds stray ever further from the nest site to- have been documented in the Biebrza valley.The most ward the end of the post-fledgingperiod. We know of no dominant forest associations include black alder (Alnus studyconcerning a raptor speciesin which the departure glutinosa),swampy birch (Betulapubescens), and peat co- on migration, the break up of the family,and subsequent niferousforests (Salici-Betuletum). Frequent anthropogen- migration have been investigated by satellite tracking. ic ecosystemsfound in the valley are pastures,cultivated Here, we report on a case concerning Greater Spotted groundsand urbanized areas.One of the greatestthreats Eagles (Aquila clanga).Available information on this spe- to the park is human modified drainagepatterns, which cies is limited, but Ivanov et al. -
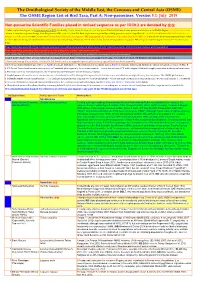
ORL 5.1 Non-Passerines Final Draft01a.Xlsx
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part A: Non-passerines. Version 5.1: July 2019 Non-passerine Scientific Families placed in revised sequence as per IOC9.2 are denoted by ֍֍ A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. English names shaded thus are taxa on BirdLife Tracking Database, http://seabirdtracking.org/mapper/index.php. Nos tracked are small. NB BirdLife still lump many seabird taxa. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. -

LEGGED BUZZARD &Lpar
]. RaptorRes. 21(1):8-13 ¸ 1987 The Raptor ResearchFoundation, Inc. NOTES ON THE BREEDING BIOLOGY OF THE LONG-LEGGED BUZZARD (Buteorufinus) IN BULGARIA ILIYA Ts. VATEV ABSTRACT.--Observationswere madeon Long-leggedBuzzard (Buteorufinus) nests in Bulgariabetween 1978-83. Egg hatchinginterval was 29-44 hr. First nestlingplumage color was dirty-white tingedbeige, cere and legs yellow; iris color changedfrom sepia at hatchingto brownish yellow-greyat fiedging. Featherswere visibleby two wk. Until two wk old, nestlingsassumed a "frozen" postureon their bellies when alarmed. Nestlingsfed unaided by the fourth wk. Fledging beganby d 49. Adults were aggressive towards humanswhile young were downy, but aggressionlessened as young got older. The Long-leggedBuzzard (Buteorufinus) is one openplains beyond. The area is grazedby sheepand cattle of Europe'sleast studiedraptors. Little detailedin- attendedby herdsmen.The landscapeis variedby scattered thorn scrub,streamside willows (Salix sp.), Carpinusorz- formation on the breeding cycle of the speciesis entalisand a smallconifer plantation (Pinus nigra). Nearest availablein the literature, especiallywith regard to arable groundis one km away. Climate is temperatecon- its nestlings(Dementiev and Gladkov 1954; Brown tinental; av. rainfall = 592.1 liter/m 2 (1981-84); alti- and Amadon 1968; Glutz et al. 1971; Harrison 1975; tude -- 7-800 m. Cramp and Simmons1980). Recently,Michev et al. RESULTS (1984) reported14 definitebreeding records for Bul- The Nest. Long-leggedBuzzards used the same garia and estimatedthe country'spopulation to be nest at Pekliuka in 1981, 1983, and (T. Michev, around 50 pairs. Also reported were noteson nest pers. comm.) 1984. A new nest, relatively slight in sites,egg size, breeding season and foodof the species. -

Winter Presence of Long-Legged Buzzard (Buteo Rufinus) in Moldova (Romania)
Travaux du Muséum National d’Histoire Naturelle © 28 décembre «Grigore Antipa» Vol. LV (2) pp. 285–290 2012 DOI: 10.2478/v10191-012-0018-6 WINTER PRESENCE OF LONG-LEGGED BUZZARD (BUTEO RUFINUS) IN MOLDOVA (ROMANIA) EMANUEL ȘTEFAN BALTAG, VIOREL POCORA, CONSTANTIN ION, LUCIAN SFÎCĂ Abstract. Long-legged Buzzard (Buteo rufinus) is a medium sized bird of prey which is known as a breeding species for Romania. In the last years it started to become a common wintering presence in the south-eastern part of Romania (Dobrogea) but it was also recorded in the more northern areas (Moldova) during the cold season. Its presence in Moldova, during the winter period, was recorded in large river valleys, with agricultural lands or grasslands and with trees or timber poles, which are used for perching. Long-legged Buzzard is a new presence for Moldavian winter seasons and it could be observed only in warm periods of winter, when the daily mean temperature is above 0°C. The wintering places are maintained not only for all winter period, but also for the next years. This behaviour could be explained by its territorial fidelity, which was recorded also in other European buzzard species during the winter period. Résumé. Buse féroce (Buteo rufinus) est un oiseau de taille moyenne de proie qui est connu comme une espèce nicheuse pour la Roumanie. Dans les dernières années, il est devenu une présence commune au cours de l’hiver au sud-est de la Roumanie (Dobroudja), mais il était également enregistré dans les régions du Nord (Moldavie) pendant la saison froide.