(Bsmi and Foki) and Glycemic Control Among Patients with Type 2 Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
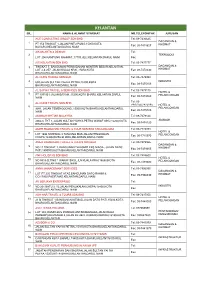
Kelantan Bil
KELANTAN BIL. NAMA & ALAMAT SYARIKAT NO.TELEFON/FAX JURUSAN ACE CONSULTING GROUP SDN BHD Tel: 09-7436625 DAGANGAN & 1 PT 153 TINGKAT 1,JALAN PINTU PONG,15000,KOTA Fax: 09-7418827 KHIDMAT BAHARU,KELANTAN,DARUL NAIM AIKON ARTS & DESIGN Tel: 2 TEKNOLOGI LOT 206 KAMPUNG RAHMAT,,17700,JELI,KELANTAN,DARUL NAIM Fax: AIR KELANTAN SDN BHD Tel: 09-7437777 DAGANGAN & 3 TINGKAT 5, BANGUNAN PERBADANAN MENTERI BESAR,KELANTAN, LOT 2 & 257, JALAN KUALA KRAI,,15050,KOTA Fax: 09-7472030 KHIDMAT BHARU,KELANTAN,DARUL NAIM AL QUDS TRAVEL SDN BHD Tel: 09-7479999 4 650,JALAN SULTAN YAHYA PETRA,15200,KOTA INDUSTRI Fax: 09-7475105 BHARU,KELANTAN,DARUL NAIM AL SAFWA TRAVEL & SERVICES SDN BHD Tel: 09-7475115 HOTEL & 5 PT 1971-B1 JALAN BAYAM,,15200,KOTA BHARU,KELANTAN,DARUL Fax: 09-7479060 PELANCONGAN NAIM Tel: 09- AL-QUDS TRAVEL SDN BHD 7475155/7475145 HOTEL & 6 9981, JALAN TEMENGGONG,,15000,KOTA BHARU,KELANTAN,DARUL PELANCONGAN Fax: 09-7475105 NAIM AMANAH IKHTIAR MALAYSIA Tel: 09-7478124 7 2002-C TKT 1,,JALAN SULTAN YAHYA PETRA WAKAF SIKU,15200,KOTA AMANAH Fax: 09-7478120 BHARU,KELANTAN,DARUL NAIM AMER RAMADHAN TRAVEL & TOUR SDN BHD TANJUNG MAS Tel: 09-7715973 HOTEL & 8 LOT 1894 SIMPANG 3 TANJUNG MAS,JALAN PENGKALAN Fax: 09-7715970 PELANCONGAN CHEPA,15300,KOTA BHARU,KELANTAN,DARUL NAIM AMER RAMADHAN TRAVEL & TOURS SDN BHD Tel: 09-7479966 DAGANGAN & 9 NO 11 TINGKAT 1, BANGUNAN TH,KOMPLEKS NIAGA , JALAN DATO' Fax: 09-7479955 KHIDMAT PATI,1500000,KOTA BHARU,KELANTAN,DARUL NAIM ANF HOLIDAYS SDN BHD Tel: 09-7488600 HOTEL & 10 NO 5515-D,TING 1 WAKAF SIKU,,JLN KUALA -

Public Involvement on Environment Issues in Kota Bharu and Jeli District, Kelantan
Journal of Social Sciences 7 (2): 175-181, 2011 ISSN 1549-3652 © 2010 Science Publications Public Involvement on Environment Issues in Kota Bharu and Jeli District, Kelantan Mohammad Ghazi Ismail and Haliza Abdul Rahman Environmental and Occupational Health Program, School of Health Sciences, University of Science Malaysia, 16150 Kubang Kerian, Kelantan Abstract: Problem statement: Environmental problems are too serious and complex to be solved through scientific approaches, technical and purely legal. Thus, public involvement with a more comprehensive vital is needed. This is because as one of the groups interested, this group can influenced and changed decision related policy legislation and policy related to environment. Public Involvement is needed in every development process as one of the positive move and proactive to create sustainable development. This study carried out in Kota Bharu and Jeli district, Kelantan, Malaysia with 390 respondent involved in each of them. Approach: Major method of study was used investigation question form. This study compared the extent to which a significant correlation (p), Mean (M) and Standard Deviation (SD) of the studied demographic factors as gender, age, race, religion, income, education and the respondent lived with the knowledge of environmental issues, environmental local environment and constraints factors involved in environmental issues at the research areas. Chi-square test used to study demographic factor association with environment issue knowledge respondent. Results: Comparison of demographic factors with knowledge of the respondents of research areas on environmental issues shows that there is relationship for respondents education level with their respective p values is 0.036 and 0.040. The relationship between demographic factors with knowledge of the local environment issues by respondent shows there is also relationship to income and education with their respective p values is 0.033, 0.019 for Kota Bharu and 0014, 0019 for Jeli. -

Summerville Industrialised Building System (IBS) Siteplan
Introducing A New Construction Method: SummerVille Industrialised Building System (IBS) Siteplan Industrialised building system (IBS) is a term used in Malaysia for a technique of construction where by components are manufactured in a controlled environment, either at site or off site, placed and assembled into construction works. The criteria we aim to achieve from an IBS methodology of building modern homes are: + Safety + Durability PHASE 1 + Economy 216 HOMES + Minimum Maintainence and Quality Assurance + Speed to completion SummerVille PHASE 2 ECRL to Kota Bharu RESIDENCES Advantages of IBS 88 HOMES 11 + IBS is able to utilized unskilled workers in construction, which in regular contruction methods may results in bad quality or defects 25 24 in buildings. It is a smart building system that is quick and requires minimal training. SUMMERVILLE 24 + IBS provides efficiency in the construction process, consequently this will shorten the construction period, which benefits RESIDENCES purchasers who have to pay progressive interest during construction period. 24 27 East Entrance 24 24 + IBS minimises the usage of timber which are not eco-friendly and might result in termite issues in future 12 14 4 West 26 15 Kuantan Entrance 19 22 Exit 17 13 24 24 20 3 16 KotaSAS 2 Access Road IBS Building Materials 5 KOTASAS CENTRAL 13 8 9 23 IBS uses concrete, a strong material which is commonly used in heavy construction that needs high load bearing capacity, such as 18 bridges, high-rise construction, retaining walls, dams, infrastructure works and airports. Rest assured your new home will be of the 21 7 highest quality building materials as well as strong. -

World Bank Document
~ Jf INTEXTATIONAL BANK FOR RECONSTRUCTION AND DEVELOPMENT DEVELOPMENT ECONOMICS DEPARTMENT Public Disclosure Authorized URBA AND REGIONAL ECONOMICS DIVISION URBAN ANW-REGIONAL REPORT NO. 72-1 ) R-72-01 DEVE)PMIET ISSJES IN THE STATES OF KELANTAN, TRENGGANU Public Disclosure Authorized AND PA HANG, MALAYSIA' JOHN C. ENGLISH SEPTEMBER 1972 Public Disclosure Authorized These materials are for internal ulse on2;7 auid are circulated to stimulate discussion and critical coxmment. Views are those of the author and should not be interpreted as reflecting the views of the World Bank. References in publications to Reports should be cleared -iith the author to protect the Public Disclosure Authorized tentative character of these papers. DEVELOPMENT ISSUES IN THE STATES OF KELANTAN, TRENGGANU AND PAHANG, MALAYSIA Table of Contents Introduction 2. Economic and Social Conditions 2.1 Population 2.2 Employment Characteristics 2.3 Incomes 2.4 Housing 2.5 Health 2.6 Transportation 2.7 Private Services 3. Economic Activity 3.1 Agriculture 3.2 Fisheries 3.3 Forestry 3.4 Manufacturing 3.5 Trade 4. Development to 1975 4.1 Agriculture and Land Development 4.2 Forestry 4.3 Projection of Agricultural and Forestry Output 4.4 Manufacturing Page 5. Conclusions 87 5.1 Transportation Links 89 5.2 Industrial Policy 92 5.3 The Role of Kuantan 96 5 .4 The Significance of Development in Pahang Tenggara 99 5.5 Racial-Balance 103 Tables and Figures 106 ~. + A5Af2;DilXlt2¢:;uessor-c.iL?-v ylixi}Ck:. -. h.bit1!*9fwI-- 1. Introduction The following report is based on the findings of a mission to Malaysia from July 3 to 25, 1972- by Mr. -

The Integration of Naqli and Aqli Knowledge in Curriculum at Universiti Sains Islam Malaysia: the Study on Student’S Internship Organizations in Kelantan, Malaysia
IJASOS- International E-Journal of Advances in Social Sciences, Vol. II, Issue 5, August 2016 THE INTEGRATION OF NAQLI AND AQLI KNOWLEDGE IN CURRICULUM AT UNIVERSITI SAINS ISLAM MALAYSIA: THE STUDY ON STUDENT’S INTERNSHIP ORGANIZATIONS IN KELANTAN, MALAYSIA Mohamed Akhiruddin Ibrahim1*, Mohammad Hikmat Shaker 2, Shahirah Sulaiman3, Azniwati Abdul Aziz 4, Nur Safura Ab. Ghaffar 5, Mohd Hisyamuddin Yusup 6 1 Senior Lecturer, Dr, Universiti Sains Islam Malaysia, MALAYSIA, [email protected] 2 Senior Lecturer, Dr, London Open Academy, UNITED KINGDOM 3 Language Teacher, Universiti Sains Islam Malaysia, MALAYSIA 4 Language Teacher, Universiti Sains Islam Malaysia, MALAYSIA 5 Language Teacher, Universiti Sains Islam Malaysia, MALAYSIA 6 Student, Universiti Sains Islam Malaysia, MALAYSIA *Corresponding author Abstract The integration of Naqli and Aqli knowledge in curriculum has been a strategic plan of Universiti Sains Islam Malaysia to become a prominent global reference institution on its instigation by year 2025. The university has introduced the term Naqli as divine knowledge; which it derives from Quran, Sunnah and references of respected books by previous religious scholars. While, the term Aqli denotes to modern knowledge that are gained through research and discoveries in the present. By having this integration in its curriculum on both of these disciplines, it has made Universiti Sains Islam Malaysia on its own as compared to other tertiary institutions. Through the integration; mandatory internship program for undergraduate students have embraced this curricular approach along with its students‟ industrial training session. The objectives for students to undergo the industrial training are for them to be familiarized with their potential job scopes, in addition, to practice the knowledge that they have learnt theoretically in the university. -

PDF File Generated From
OCCASION This publication has been made available to the public on the occasion of the 50th anniversary of the United Nations Industrial Development Organisation. DISCLAIMER This document has been produced without formal United Nations editing. The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations Industrial Development Organization (UNIDO) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries, or its economic system or degree of development. Designations such as “developed”, “industrialized” and “developing” are intended for statistical convenience and do not necessarily express a judgment about the stage reached by a particular country or area in the development process. Mention of firm names or commercial products does not constitute an endorsement by UNIDO. FAIR USE POLICY Any part of this publication may be quoted and referenced for educational and research purposes without additional permission from UNIDO. However, those who make use of quoting and referencing this publication are requested to follow the Fair Use Policy of giving due credit to UNIDO. CONTACT Please contact [email protected] for further information concerning UNIDO publications. For more information about UNIDO, please visit us at www.unido.org UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Tel: (+43-1) 26026-0 · www.unido.org · [email protected] 16058 • SMALL SCALE INDUSTRIAL DEVELOPMENT IN MALAYSIA: THE CASE OF THE 'NURSERY FACTORY SCHC.ME' IN PENGKALAN CHtPA, KELANTAN by Anuwar Ali & Ismail M. -

Senarai Premis Penginapan Pelancong : Kelantan 1
SENARAI PREMIS PENGINAPAN PELANCONG : KELANTAN BIL. NAMA PREMIS ALAMAT POSKOD DAERAH 1 Crystal Lodge 124, Jalan Che Su 15000 KOTA BHARU 2 Sutera Inn Prima Jalan Dusun Muda, Off Jalan Bayam 15200 KOTA BHARU 3 KESEDAR HOTEL TRAVEL & TOURS Jalan Kesedar Inn 18300 Gua Musang 4 Suria Hotel Lot 254-256, Jalan Padang Garong 15000 KOTA BHARU 5 H.B Village Resort Pantai Cahaya Bulan 15350 KOTA BHARU 6 Hotel Usaha 187, Jalan Pantai 18300 Gua Musang 7 Grand Riverview Hotel Jalan Post Office Lama 15000 KOTA BHARU 8 Renaissance Kota Bharu Hotel Kota Sri Mutiara, Jalan Sultan Yahya Petra 15150 KOTA BHARU 9 Paloh Inn Wisma Nordin, Lot 74, Kg.Paloh, Pintu Geng 15050 KOTA BHARU 10 Dynasty Inn 2865-D & E, Jalan Sultanah Zainab 15050 KOTA BHARU 11 Irama Timur Resort House 6800-D, Jalan Bayam Lama 15200 KOTA BHARU 12 Sabrina Court Hotel Lot 171-181, Jalan Padang Garong 15000 KOTA BHARU 13 Sutera Calan Lot 1911, 1912, 1957, Kg.Bandar Taway 16300 Bachok 14 d'Mawar Chalet Taman Tasik Sri 17500 Tanah Merah 15 Fully Inn 75, Jalan Pekan Lama 18300 Gua Musang 16 Sudara Beach Resort Lot 128, Mukim Telong,Jln Tok Bali 16300 Bachok 17 Z & H Inn Lot 2731, Paya Rambai, Jln. Bayam 15200 KOTA BHARU 18 D Muara Chalet Lot 3067 Kg Sg.Dua Melati Repek 16300 Bachok 19 Flora Place Lot 202 Jalan Kebun Sultan 15350 KOTA BHARU 20 Pasir Belanda Resort Kg Lipat Sanggul, Kg Banggol 15350 KOTA BHARU 21 Ridel Hotel Kota Bharu Lot A-05-01, Jalan Pasar Lama, Seksyen 9 15000 KOTA BHARU 22 Royal Guest House Lot 440 & 443, Lot 448-452, Jln Hilir Kota 15300 KOTA BHARU 23 Bayu Melawi Chalet Lot 3066, Kg. -

Bahagian Penguatkuasaan Dan Kawalan Alamat Cawangan
BAHAGIAN PENGUATKUASAAN DAN KAWALAN ALAMAT CAWANGAN: Pengarah Bahagian Penguatkuasaan Dan Kawalan Kementerian Dalam Negeri Aras 4, Blok 1, Kompleks KDN Taman Setia Tropika, Kempas 81200 Johor Bahru JOHOR No telefon : 07-2344667 No faksimili : 07-2344662 Pengarah Bahagian Penguatkuasaan Dan Kawalan Kementerian Dalam Negeri Aras 3, Kompleks KDN WPKL No.69, Jalan Sri Hartamas 1 50480 KUALA LUMPUR No telefon : 03-6201 2099 No faksimili : 03-6201 2104 Pengarah Bahagian Penguatkuasaan Dan Kawalan Kementerian Dalam Negeri Tingkat 05-02, Plaza Masalam No.2, Jalan Tengku Ampuan Zabedah E9/E, Seksyen 9 40100 Shah Alam SELANGOR No telefon : 03-55190349 No faksimili : 03-55124016 Pengarah Bahagian Penguatkuasaan Dan Kawalan Kementerian Dalam Negeri Tingkat 4 & 5, Bangunan Tabung Haji Kompleks Terminal 1 , Jalan Lintang 70000 Seremban NEGERI SEMBILAN. No telefon : 06-7611152 No faksimili : 06-7620402 Pengarah Bahagian Penguatkuasaan Dan Kawalan Kementerian Dalam Negeri Aras 2, Kompleks KDN Bandar Indera Mahkota 25200 Kuantan PAHANG No telefon : 09-5734466 No faksimili : 09-5739991 Pengarah Bahagian Penguatkuasaan Dan Kawalan Kementerian Dalam Negeri Aras 3, Blok Pentadbiran Kompleks KDN Jalan Seri Negeri, Ayer Keroh, 75450 MELAKA No telefon : 06-2326068 No faksimili : 06-2326070 Pengarah Bahagian Penguatkuasa Dan Kawalan Kementerian Dalam Negeri Aras 3, Kompleks KDN Perak Persiaran Meru Utama, Bandar Meru Raya, 30020 Ipoh, PERAK No telefon : 05-5279002 No faksimili : 05-5278001 Pengarah Bahagian Penguatkuasaan Dan Kawalan Kementerian Dalam Negeri Aras 3, Bangunan Kementerian Dalam Negeri Kompleks Pentadbiran Kerajaan Persekutuan Bandar Mu'adzam Shah, Anak Bukit 06550 Alor Setar, KEDAH No telefon : 04-7358454 No faksimili : 04-7355653 Pengarah Bahagian Penguatkuasaan Dan Kawalan Kementerian Dalam Negeri No. -

Peta Panduan Jalan KELANTAN Ibu Negeri
PETUNJUK Peta Panduan Jalan KELANTAN Ibu Negeri Daerah 101º 20’E 1 101º 30’E 2 101º 40’E 3 101º 50’E 4 102º 00’E 5 102º 10’E 6 102º 20’E 7 102º 30’E 8 102º 40’E Bazar Tengku Anis Pekan Hentian Bas ke PCB GG Garden Hostel Istana Nasi R JLN. DUSUN RAJA N A Batu Ulam Payang Serai LAUT CHINA A Tourism Khalifa Jln. Maahad R Apartment Malaysia Star Family Kota Timur Hostel Kg. Kraf Tambatan Muzium Sempadan Antarabangsa di Raja Royal Tangan & E’ E Inn Perang Muzium Masjid Guesthouse Muzium R Sri Chiang Mai Islam Muhammadi Feri ke Menara Jln. HilirKraf Kota SELATAN Kampung Tinjau Ideal China Town Laut Pasar Malam Guesthouse Padang Merdeka Istana Oriental Kopitiam Jahar R Kampung Pantai 6º 15’N Pantai Seri Tujuh 6º 15’N Sempadan Negeri Ridel Jln. Tengku Besar Istana Jln. Sekolah Merbau Crown Garden Suri Homestay Pengkalan Kok Majid Jetty Money Changer Dynasty Balai Besar Arena Seni Suara Wat Machimmaram KFC JLN. KEBUN SULTAN Burung Indah Pantai Juite Pantai Cahaya Bulan Timur R Mydin Inn Sempadan Daerah Pasar Besar Pengkalan Kubor Bazar JLN. PINTU PONG Jln. Pasar Lama Siti Khadijah Pantai Kuala Pak Amat Jln. Tengku Chik Buluh Kubu R Jln. Seri Cemerlang Wat Mai Suvankhiri 134 Tumpat Mc’Donalds JLN. BULUH KUBU MGU Pantai Sabak B Muzdalfa Fried R Firdaus B CELCOMWat Centre Photivihan TUMPAT Jalanraya Persekutuan Jln. Tg. Putera Jln. Tok Hakim Chicken Flora Temenggong Place KB Mutiara Inn Sultan Ismail Petra Airport Parkson/ Giant Mydin R FourMakam Season Tok Janggut Suntwo R MAXIS Centre Sri Cemerlang Grand The Riverview Hostel Pengkalan Chepa KFC Store Sabrina KOTA BHARU R Court Wakaf Bharu Nombor Jalan Persekutuan Pizza JLN. -

Lampiran.Pdf
LAMPIRAN i. JADUAL HIERARKI BANDAR Dasar Perbandaran Negara Kedua Semenanjung Malaysia dan Wilayah Persekutuan Labuan Jadual Hierarki dan Karakter Utama Bandar Bagi Semenanjung Malaysia dan Wilayah Persekutuan Labuan Negeri Hierarki Bandar Bil. Bandar Karakter Utama Bandar Wilayah Persekutuan Bandar Global 1 Kuala Lumpur Kuala Lumpur Wilayah Persekutuan Bandar Global 2 Putrajaya Putrajaya Wilayah Persekutuan Bandar Wilayah 3 Labuan (Kes Khas) Labuan Bandar Tempatan 4 Kampung Bebuloh 5 Kampung Ganggarak 6 Kampung Layang-Layangan 7 Kampung Simpang Tamu Perlis Bandar Negeri 8 Kangar Bandar Tempatan 9 Arau Bandar Diraja 10 Beseri 11 Kuala Perlis 12 Lembah Chuping Bandar Pelabuhan (Darat) 13 Padang Besar Bandar Sempadan 14 Pauh Putra Kedah Bandar Negeri 15 Alor Setar Bandar Utama 16 Kulim Bandar Perindustrian 17 Sungai Petani Bandar Tempatan 18 Baling 19 Bandar Baharu 20 Bandar Bukit Kayu Hitam 21 Bedong 22 Changlun 23 Guar Cempedak 24 Gurun Bandar Perindustrian 25 Jitra 26 Kuala Kedah Bandar Pelancongan 27 Kuala Ketil 28 Kuala Nerang 29 Kuah Bandar Pelancongan 30 Merbok 31 Padang Mat Sirat 32 Padang Serai 33 Pendang 34 Pokok Sena 35 Serdang 36 Sik 37 Sungai Karangan 38 Sungai Kob 39 Sungai Lalang 40 Tikam Batu 41 Yan Pulau Pinang Bandar Wilayah 42 George Town Bandar Utama 43 Balik Pulau 44 Batu Kawan - Cassia 45 Bayan Baru 46 Bukit Mertajam 47 Butterworth Bandar Perindustrian 48 Kepala Batas 49 Nibong Tebal Bandar Tempatan 50 Alma 51 Ayer Itam 52 Bagan Ajam 53 Bandar Bertam L1 Dasar Perbandaran Negara Kedua Semenanjung Malaysia dan Wilayah Persekutuan Labuan Jadual Hierarki dan Karakter Utama Bandar Bagi Semenanjung Malaysia dan Wilayah Persekutuan Labuan Negeri Hierarki Bandar Bil. -

Senarai Klinik Panel (Ob) Perkeso Yang Berkelayakan* (Kelantan)
SENARAI KLINIK PANEL (OB) PERKESO YANG BERKELAYAKAN* (KELANTAN) BIL NAMA KLINIK ALAMAT KLINIK NO. TELEFON KOD KLINIK NAMA DOKTOR 1 KLIIK GOH 3579, JALAN ISMAIL, 15000 KOTA BHARU, KELANTAN. 09-7481254 K91006K DR. GOH KHOON GEE 2 KLINIK FOO SDN BHD 3597, JALAN ISMAIL 15000, KOTA BHARU, KELANTAN. 09-7483518 K91008Z DR. FOO MENG HOW KLINIK PERDANA 160, BANGUNAN PKINK, PENGKALAN CHEPA, 3 (CAWANGAN PENGKALAN 09-7738916 K91016Z DR. HILLALUDDIN B. HASHIM 16100 KOTA BHARU, KELANTAN. CHEPA) 40, JALAN SUARA MUDA, 15000 KOTA BHARU, 4 KLINIK PERDANA 09-7444455 K91017X DR. EZANEE ISAHAK KELANTAN. 438 H, JALAN LUBOK SETUL, 17200 RANTAU PANJANG, 5 KLINIK PERDANA / DR. YASIMAN 09-7950288 K91018V DR. YASIMAN B. HAJI YUSOFF KELANTAN. LOT 434/444, JALAN SULTAN YAHYA PETRA, 6 KLINIK ZAITUN 09-7486337 K91030K DR. ZAITUN BINTI TAIB WAKAF SIKU, 15200 KOTA BHARU, KELANTAN. 7 KLINIK CHONG 3576, JALAN ISMAIL, 15000 KOTA BHARU, KELANTAN. 09-7446496 K91035M DR.CHONG LUM CHEONG 55-A JALAN PASAR LAMA, 17000 PASIR MAS, 8 KLINIK JO 09-7909879 K91037A DR.JOGINDER SINGH RAKHRA KELANTAN. 2981-A, JALAN PADANG GARONG, 15000 KOTA 9 KLINIK WEE 09-7444140 K91038Y DR.WEE TIANG GOH BHARU, KELANTAN. LOT 3099, JALAN PADANG TEMBAK, 16100 KOTA 10 KLINIK KELUARGA 09-7742637 K91040Z DR. AZLAM B. MOHD ZAIN BHARU, KELANTAN. PT 361, KEDAI MELOR, 16400 KOTA BHARU, 11 KLINIK HAJI RAZALI & X-RAY 09-7536666 K91046Y DR. HAJI RAZALI HAMZAH KELANTAN. 58A, JALAN PEKAN LAMA, 18300 GUA MUSANG, 12 KLINIK RAJU 09-9121243 K92004F DR. RENGARAJU A/L GOVINDASAMY KELANTAN. 1520, JALAN TENGKU ZAINAL ABIDIN, 18000 KUALA 13 KLINIK SHAFEE 09-9666626 K92005A DR. -

Geology of the Batu Melintang-Sungai Kolok Transect Area Along the Malaysia – Thailand Border
GEOLOGY OF THE BATU MELINTANG-SUNGAI KOLOK TRANSECT AREA ALONG THE MALAYSIA – THAILAND BORDER By The Malaysian and Thai Working Groups A joint project carried out by Minerals and Geoscience Department Malaysia and Department of Mineral Resources, Thailand The Malaysia-Thailand Border Joint Geological Survey Committee (MT-JGSC) 2006 - ii - PREFACE This report together with its accompanying geological map on the scale 1:250,000 is the result of close cooperation between the Minerals and Geoscience Department Malaysia, and the Department of Mineral Resources, Thailand in resolving problems related to cross border geological correlation between Malaysia and Thailand. Fieldwork was carried out separately (July – August 2001) in the Batu Melintang and Sungai Kolok areas and then jointly checked (5-11 April 2002) by geoscientists from both Malaysia and Thailand, covering a total area of about 1,350 square kilometres along the common border. Problems on the discontinuity of time rock unit boundaries between the various rocks found in both sides of the common border areas have been satisfactorily resolved. Potential mineral deposits occur along the border areas but the present security situation, as well as environmental issues does not encourage the exploitation of these mineral deposits. Future increase in the price of minerals coupled with advances in mining technology may facilitate joint exploitation of these mineral deposits. Dr. Chu Ling Heng Mr. Somsak Potisat Director General Director General Minerals and Geoscience Department Department