Incidence and Characteristics of Kidney Stones in Patients on Ketogenic Diet: a Systematic Review and Meta-Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Of Treatment of Hyperuricemia on Effect
Faculty of Medicine Institutional Review Board (IRB) • Research Proposal Form This section is for Official Use Only Reference Code: Date of application (dd/mm/yyyy): NCT ID: Not yet assigned 15/09/2020 Revision 1: 10/12/2020 20/02/2021 Revision 2: This section is for the applicant to fill. • About 2000 word limit applies, excluding references. • Use Times New Romans Font, size 11 and adjust line spacing to 1.5 all through the application form • Do not CAPITALIZE all words Part 1: General Master Degree b. MD c. Independent Research/Project 1.1 Applicant Name (responsible for all correspondences and accuracy of data): Department: Nephrology Mohamed Ragab Eldremi email address: [email protected] Mobile Phone: 01114430050 EFFECT OF TREATMENT OF HYPERURICEMIA ON Home Phone: 0863553849 PROGRESSION OF DIABETIC NEPHROPATHY IN PATIENTS WITH TYPE 2 DIABETES MELLITUS AND STAGE 3 CHRONIC KIDNEY DISEASE. Assiut Medical School Research Proposal Form 1 Faculty of Medicine Institutional Review Board (IRB) 1.2 English Title of research project: EFFECT OF TREATMENT OF HYPERURICEMIA ON PROGRESSION OF DIABETIC NEPHROPATHY IN PATIENTS WITH TYPE 2 DIABETES MELLITUS AND STAGE 3 CHRONIC KIDNEY DISEASE. 1.3 Do you need funding from Assiut Medical School Grants Office? Yes No (If no, skip and delete Part 4) Mention other sponsoring agent(s) if any: ………………no…………………………... Part 2: Research Details Assiut Medical School Research Proposal Form 2 Faculty of Medicine Institutional Review Board (IRB) 2.1 Background (Research Question, Available Data from the literature, Current strategy for dealing with the problem, Rationale of the research that paves the way to the aim(s) of the work). -

A Study of the Correlation Between Altered Blood Glucose and Serum Uric Acid Levels in Diabetic Patients
Jebmh.com Original Research Article A Study of the Correlation between Altered Blood Glucose and Serum Uric Acid Levels in Diabetic Patients Simbita A. Marwah1, Mihir D. Mehta2, Ankita K. Pandya3, Amit P. Trivedi4 1Associate Professor, Department of Biochemistry, Parul Institute of Medical Sciences & Research, Vadodara, Gujarat, India. 2Associate Professor, Department of Biochemistry, Parul Institute of Medical Sciences and Research, Vadodara, Gujarat, India. 3Student, Department of Biochemistry, Pramukhswami Medical College, Karamsad, Gujarat, India. 4Associate Professor, Department of Biochemistry, Pramukhswami Medical College, Karamsad, Gujarat, India. ABSTRACT BACKGROUND The prevalence of diabetes mellitus ranges from 0.4 - 3.9% in rural areas to 9.3 - Corresponding Author: 16.6% in urban areas, in India. Diabetes causes long term dysfunction of various Dr. Mihir Mehta, Associate Professor, organs like heart, kidneys, eyes, nerves, and blood vessels. Hyperuricemia is Department of Biochemistry, defined as serum uric acid concentration in excess of urate solubility. In non- Parul Institute of Medical Sciences and diabetic subjects, an elevated level of uric acid has been shown to be an Research, Vadodara, Gujarat, India. independent predictor of coronary heart disease and total mortality. Also elevated E-mail: [email protected] levels of uric acid is a risk factor for peripheral arterial disease. DOI: 10.18410/jebmh/2020/268 METHODS Financial or Other Competing Interests: This is a cross sectional study conducted over a period of 1 year. 565 individuals None. visiting the routine health check-up were included in the study. Serum uric acid, glycated haemoglobin (HbA1c) and glucose were estimated on Siemens Dimension How to Cite This Article: auto analyser. -

Relationship Between Diabetes Mellitus and Serum Uric Acid Levels
Int. J. Pharm. Sci. Rev. Res., 39(1), July – August 2016; Article No. 20, Pages: 101-106 ISSN 0976 – 044X Review Article Relationship between Diabetes Mellitus and Serum Uric Acid Levels J. Sarvesh Kumar*, Vishnu Priya V1, Gayathri R2 *B.D.S I ST YEAR, Saveetha Dental College, 162, P.H road, Chennai, Tamilnadu, India. 1Associate professor, Department of Biochemistry, Saveetha Dental College, 162, P.H Road, Chennai, Tamilnadu, India. 2Assistant professor, Department of Biochemistry, Saveetha Dental College, 162, P.H Road, Chennai, Tamilnadu, India. *Corresponding author’s E-mail: [email protected] Accepted on: 03-05-2016; Finalized on: 30-06-2016. ABSTRACT The aim of the study is to review the association between diabetes Mellitus and serum uric acid levels. The objective is to review how uric acid level is related to diabetes Mellitus. Diabetes is an increasingly important disease globally. New data from IDF showed that there are 336 million people with diabetes in 2011 and this is expected to rise to 552 million by 2030. It has been suggested that, diabetic epidemic will continue even if the level of obesity remains constant. The breakdown of foods high in protein into chemicals known as purines is responsible for the production of uric acid in the body. If there is too much of uric acid in the body it causes variety of side effects. Thus identifying risk factors of serum uric acid is required for the prevention of diabetes. The review was done to relate how serum uric acid level is associated with the risk of diabetes. -

Can Hyperuricemia Predict Glycogen Storage Disease (Mcardle's Disease) in Rheumatology Practice? (Myogenic Hyperuricemia)
Clinical Rheumatology (2019) 38:2941–2948 https://doi.org/10.1007/s10067-019-04572-8 CASE BASED REVIEW Can hyperuricemia predict glycogen storage disease (McArdle’s disease) in rheumatology practice? (Myogenic hyperuricemia) Döndü Üsküdar Cansu1 & Bahattin Erdoğan2 & Cengiz Korkmaz1 Received: 25 March 2019 /Revised: 17 April 2019 /Accepted: 18 April 2019 /Published online: 1 May 2019 # International League of Associations for Rheumatology (ILAR) 2019 Abstract Gout disease is an inflammatory arthritis that arises due to the accumulation of monosodium urate crystals (MSU) around the joints and in tissues. Clinical manifestation of metabolic diseases leading to secondary hyperuricemia most predominantly occurs in the form of gouty arthritis. Hyperuricemia and gout may develop during the course of glycogen storage diseases (GSD), particularly in GSD type I, which involves the liver. On the other hand, during the course of GSD type V (GSDV, McArdle’s disease), which merely affects the muscle tissue due to the deficiency of the enzyme myophosphorylase, hyperuricemia and/or gout is rarely an expected symptom. These patients may mistakenly be diagnosed as having idiopathic hyperuricemia and associated gout, leading to the underlying secondary causes be overlooked and thus, diagnostic delays may occur. In this case report, we present a premenopausal female patient who experienced flare-ups of chronic arthritis while on disease-modifying antirheumatic drugs and intraarticular steroids due to a diagnosis of undifferentiated arthritis. The patient was initially suspected of having gouty arthritis because elevated concentrations of uric acid were incidentally detected, but then, a diagnosis of asymptomatic GSDV was made owing to elevated concentrations of muscle enzymes during colchicine use. -

Biomarkers in Serum, Uric Acid As a Risk Factor for Type 2 Diabetes Associated with Hypertension
Online - 2455-3891 Vol 9, Issue 2, 2016 Print - 0974-2441 Research Article BIOMARKERS IN SERUM, URIC ACID AS A RISK FACTOR FOR TYPE 2 DIABETES ASSOCIATED WITH HYPERTENSION TRIPATHI GK1*, RACHNA SHARMA2, MANISH KUMAR VERMA3, PREETI SHARMA4, PRADEEP KUMAR4 1Department of Medicine, Hind Institute of Medical Sciences, Barabanki, Uttar Pradesh, India. 2Department of Biochemistry, TSM Medical College and Hospital, Lucknow, Uttar Pradesh, India. 3Department of Biochemistry, Integral Institute of Medical Sciences & Research, Lucknow, Uttar Pradesh, India. 4Department of Biochemistry, Santosh Medical College & Hospital, Santosh University, Ghaziabad, Uttar Pradesh, India. Email: [email protected] Received: 27 January 2016, Revised and Accepted: 30 January 2016 ABSTRACT Objectives: Uric acid (UA) is the end product of purine metabolism in humans. UA is the final oxidation product of purine catabolism and has been implicated in diabetes mellitus (DM) as well as in hyperlipidemias. Hyperuricemia can cause serious health problems including renal insufficiency. Hyperuricemia is associated with many diseases including hypertension (HTN), DM, hypertriglyceridemia, and obesity. The aim was to determine the serum UA (SUA) level in Patients of Type 2 DM with HTN. Methods: Out of 100 samples, 50 were found as cases of Type 2 diabetic with HTN, and the 50 control samples were without Type 2 diabetic HTN. Results: SUA, glycosylated hemoglobin, and low-density lipoprotein of male and female cases of Type 2 DM with HTN compared to control were (p<0.05) highly significant and also serum triglycerides and total cholesterol of both sex groups of Type 2 DM with HTN compared to control were found to be (p<0.05) highly significance. -

Evaluation of Alteration of Serum Uric Acid Level in Hypothyroid and Hyperthyroid Patients in a Tertiary Care Hospital
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2016): 79.57 | Impact Factor (2015): 6.391 Evaluation of Alteration of Serum Uric Acid Level in Hypothyroid and Hyperthyroid Patients in a Tertiary Care Hospital Dr. Sayari Banerjee1, Dr. Jayati Roy Choudhury2 1, 2Department of Biochemistry, College of Medicine and Sagore Dutta Hospital Abstract: It has been demonstrated by several studies that thyroid function can affect almost all metabolic activity. Both hypo and hyperthyroidism have potentially fatal systemic manifestations. Hypothyroidism is a clinical syndrome resulting from a deficiency of thyroid hormones which, in turn, results in a generalized slowing down of all metabolic processes. Hypothyroidism is associated with many biochemical abnormalities including increased uric acid levels. Reason behind hyperuricaemia in patients with hyperthyroid status, who are expected to have a higher renal clearance of uric acid may be the increased uric acid production secondary to increased overall metabolism in hyperthyroid patients. According to null hypothesis there is no significant relationship between thyroid status and uric acid level. Justification of this present hospital based non interventional case control study was designed to find any association between uric acid level with hypothyroidism and hyperthyroidism in comparison to normal control. In our study we find that 50 patients of primary hypothyroidism also suffers from hyperuricemia whereas there is also significantly high uric acid level found in primary 31primary hyperthyroid patients in comparison to euthyroid population. Keywords: Hypothyroid, Hyperthyroid, Euthyroid, Hyperuricemia 1. Introduction hypo/hyperthyroidism, any substance abuse, chronic alcoholic, any drug intake that affect renal and liver It was asserted by various studies that hypothyroidism is function, Chronic inflammatory disease. -

Comparative Study of ALT, AST, GGT & Uric Acid Levels in Liver Diseases
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861. Volume 7, Issue 5 (May.- Jun. 2013), PP 72-75 www.iosrjournals.org Comparative Study of ALT, AST, GGT & Uric Acid Levels in Liver Diseases Dr. G. Vijaya Benerji1, M. Farid Babu 2, Rekha Kumari. D. 2, Aditi Saha3 1Associate professor, Department of Biochemistry, Konaseema Institute of Medical Sciences Research Foundation, Amalapuram, A.P. India. 2Assistant professor, Department of Biochemistry, Konaseema Institute of Medical Sciences & Research Foundation, Amalapuram, A.P. India. 3PG student,Department of Biochemistry,Konasema Institute of Medical Sciences and Research Foundation, Amalapuram, A.P, India Abstract:Hepatic injury is associated with distortion of the metabolic function. Hepatic disease/Cirrhosis of liver can be evaluated by biochemical analysis of serum tests, includes levels of serum Alanine and Aspartate amino transferases, Alkaline Phosphatase, and also by Uric Acid estimation. The present study was continued to assay liver associated enzymes on patients with cirrhosis of liver, Amoebic liver abscess and hepatitis and to find out the comparative levels of enzymes and uric acid among the groups. In this study total 80 male subjects ( 25 healthy controls and 55 patients as case groups) aged between 20 to 60. yrs. Was enrolled. One case group consists 25 male patients(cirrhosis of liver) and second case group consists of 15 male patients (Amoebic liver abscess disease) and third case group consists of 15 male patients ( Hepatitis ) suffering from corresponding diseases ad controls group subjects are 25 in number. ALT, AST, ALP,GGT and uric acid levels are estimated in the above groups by standard methods. -

Lung Function Decline Is Associated with Serum Uric Acid in Korean
www.nature.com/scientificreports OPEN Lung function decline is associated with serum uric acid in Korean health screening individuals Kyung‑Min Ahn1, Suh‑Young Lee1, So‑Hee Lee1,2, Sun‑Sin Kim1,2* & Heung‑Woo Park1,3,4* We performed a retrospective cohort study of 19,237 individuals who underwent at least three health screenings with follow‑up periods of over 5 years to fnd a routinely checked serum marker that predicts lung function decline. Using linear regression models to analyze associations between the rate of decline in the forced expiratory volume in 1 s (FEV1) and the level of 10 serum markers (calcium, phosphorus, uric acid, total cholesterol, total protein, total bilirubin, alkaline phosphatase, aspartate aminotransferase, creatinine, and C‑reactive protein) measured at two diferent times (at the frst and third health screenings), we found that an increased uric acid level was signifcantly associated with an accelerated FEV1 decline (P = 0.0014 and P = 0.037, respectively) and reduced FEV1 predicted % (P = 0.0074 and P = 8.64 × 10–7, respectively) at both visits only in non‑smoking individuals. In addition, we confrmed that accelerated forced vital capacity (FVC) and FEV1/FVC ratio declines were observed in non‑smoking individuals with increased serum uric acid levels using linear mixed models. The serum uric acid level thus potentially predicts an acceleration in lung function decline in a non‑smoking general population. Abbreviations FEV1 Forced expiratory volume in 1 s FVC Forced vital capacity LF Lung function Lung function (LF) gradually declines over time with wide inter-individual variations 1,2. -

Internet Journal of Medical Update 2009 July;4(2):36-41 Internet Journal of Medical Update
Internet Journal of Medical Update 2009 July;4(2):36-41 Internet Journal of Medical Update Journal home page: http://www.akspublication.com/ijmu Original Work Dynamic Changes in Biochemical Markers of Renal Function with Thyroid Status – A Study in Indian Population Dr. Devika Tayal MD, Dr. Ranjna Chawla PhD, Dr. Sarika Arora MD, Dr. Vinod K Gupta MD, Mr. Jagdeep S Sohi MSc and Dr. Venkatesan MallikaΨ MD Department of Biochemistry, G B Pant Hospital, New Delhi, India (Received 22 October 2008 and accepted 31March 2009) ABSTRACT: Thyroid dysfunction is known to cause significant changes in glomerular filtration rate. The present cross-sectional study was performed to evaluate the changes in biochemical markers of renal function in hypothyroid subjects before and after treatment. Thyroid function tests (T3, T4 and TSH levels) were assayed in 385 subjects. Based on TSH levels, subjects were classified as euthyroid (n=198), sub-clinical hypothyroid (n=98; TSH 6.1 to 19.9 μIU/ml) and overt hypothyroid (n=89; TSH ≥ 20 μIU/ml, abnormally low T4 levels). Forty-eight hypothyroid patients were re-evaluated after 3 months of thyroxine replacement therapy. Renal function tests were carried out in all subjects and statistically analyzed. Serum creatinine was significantly increased in sub- clinical and overt hypothyroid groups as compared to euthyroid subjects. Serum creatinine showed a significant negative correlation with T3 &T4 levels in overt group(r = -0.372 and r = - 0.371), whereas a positive correlation was observed with TSH (r=0.283). Uric acid levels were significantly increased in the overt group as compared to euthyroid subjects. -
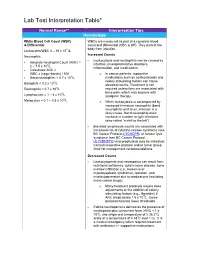
Lab Test Interpretation Table*
Lab Test Interpretation Table* Normal Range** Interpretation Tips Hematology White Blood Cell Count (WBC) WBCs are measured as part of a complete blood & Differential count and differential (CBC & diff). They protect the body from infection. Leukocytes/WBC 4 – 10 x 109 /L Increased Counts Neutrophils - Leukocytosis and neutrophilia can be caused by Absolute Neutrophil Count (ANC) = - infection, myeloproliferative disorders, 2 – 7.5 x 109/L inflammation, and medications. - Calculated ANC = WBC x (segs+bands) / 100 o In cancer patients, supportive - Band neutrophils: < 0.7 x 109/L medications such as corticosteroids and 9 colony stimulating factors can cause Basophils < 0.2 x 10 /L elevated counts. Treatment is not Eosinophils < 0.7 x 109/L required unless they are associated with 9 bone pain, which may improve with Lymphocytes = 1 – 4 x 10 /L analgesic therapy. 9 Monocytes = 0.1 – 0.8 x 10 /L o When leukocytosis is accompanied by increased immature neutrophils (band neutrophils) and fever, infection is a likely cause. Band neutrophils often increase in number to fight infections (also called “a shift to the left”). - Elevated lymphocyte counts are associated with increased risk of cytokine-release syndrome (see BC Cancer Protocol LYCHOPR) or tumour lysis syndrome (see BC Cancer Protocol ULYVENETO) and prophylaxis may be indicated. Consult respective protocol and/or tumor group chair for management recommendations. Decreased Counts - Leukocytopenia and neutropenia can result from nutritional deficiency, autoimmune disease, bone marrow infiltration (i.e., leukemia or myelodysplastic syndrome), radiation, and myelosuppression due to medications (including many cancer drugs). o Many treatment protocols require dose adjustments or the addition of colony stimulating factors (e.g., filgrastim) if ANC drops below 1.5 x 109/L. -

Understanding Gout and Your Kidneys
UNDERSTANDINGUnderstanding GOUT ANDYour YOUR KIDNEYS Hemodialysis Access Options UNDERSTANDING GOUT AND YOUR KIDNEYS WHAT IS GOUT? Gout happens if a substance called uric acid gets too high in your blood. Gout is a common form of arthritis. Everyone has small amounts of uric It can cause pain, swelling, and acid in their blood. It comes from redness in your joints. Gout usually two places—the normal breakdown begins in the big toe, but can strike of your body’s cells and certain other joints such as the ankle, knee, foods. At normal levels, uric acid wrist, fingers, or elbow. Usually does not cause any damage. But if only one joint is affected, but some the levels get too high, it can form people may have gout in more than sharp crystals that build up in your one joint, especially if it is not found body’s joints and cause pain. The and treated. medical term for high blood urate levels is hyperuricemia. DID YOU KNOW? • About 8.3 million U.S. adults HOW DOES URIC ACID (3.9 percent) are living with CAUSE GOUT? gout today and up to 10% of people worldwide may have Whenever you eat or drink gout. Gout is the most common something, your body pulls out the form of arthritis. good stuff like vitamins, and gets rid of the waste. One of those • Gout puts you at risk for kidney waste products is uric acid. It is disease. Up to 20% percent of made when your body breaks down people with gout have mild to purines, which is found in certain moderate kidney disease. -

Original Research Article
TWAN ME I DI CH C Journal of Chitwan Medical College 2019;9(30):6-10 A F L O C L O Available online at: www.jcmc.cmc.edu.np A L N L E R G U E O J JCMC ESTD 2010 ORIGINAL RESEARCH ARTICLE SERUM URIC ACID IN PATIENTS WITH ABNORMAL THYROID FUNCTIONS: A CROSS-SECTIONAL STUDY Kushal Bhattarai1,*, Nilu Manandhar2 1Department of Biochemistry, College of Medical Sciences and Teaching Hospital, Bharatpur, Chitwan, Nepal. 2Department of Physiology, College of Medical Sciences and Teaching Hospital, Bharatpur, Chitwan, Nepal. Received: 10 Nov, 2019 ABSTRACT Accepted: 27 Nov, 2019 Background: Hyperuricemia, a pathologic consequence of over-production or under-excre- Published: 27 Dec, 2019 tion of uric acid, is a common biochemical picture in thyroid dysfunctions. This study was designed to determine the serum uric acid levels and the prevalence rates of hyperuricemia Key words: Hyperthyroidism; Hypothyroidism; Se- in thyroid dysfunctions. rum uric acid; Hyperuricemia. Methods: A cross-sectional study was conducted at College of Medical Sciences and Teaching *Correspondence to: Kushal Bhattarai, Department of Hospital, Chitwan in which Serum uric acid concentrations of male and female participants, 30 years or older with the diagnosis of hypothyroidism or hyperthyroidism were estimated. Biochemistry, College of Medical Science and Teaching Hyperuricemia was defined as uric acid levels >6.8mg/dL (males) and >6.0 mg/dL (females). Hospital, Bharatpur, Chitwan, Nepal. Descriptive statistics using frequency with percentage and inferential statistics using para- Email: [email protected] metric and non-parametric tests were used. DOI:https://doi.org/10.3126/jcmc.v9i4.26891 Results: In the total 249 participants, the prevalence of hyperuricemia was 30.5% (32.9% in hyperthyroid and 26.1% in hypothyroid patients).