Protocol for a Multi-Site Pilot and Feasibility Randomised Controlled Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shoulder Pain Advice and Exercises
Shoulder Pain Advice and Exercises 0523 - Oct 2018 (v1.2) April 2018 April 2021 Please note that if your shoulder pain started after a recent trauma please Simple Exercises contact your GP. Please complete all exercises below as your symptoms allow; aim for no more than 10-15 repetitions of each exercise, 2-3 times a day. Some exercises may cause This booklet has been produced by senior physiotherapists working for discomfort but should not cause significant pain. If your symptoms are not DynamicHealth. It offers simple advice and exercises to help you safely manage improving in 6 - 8 weeks or are worsening please call our Physio Advice Line for your shoulder problem, often the right advice and exercises are all that is needed. further support on 0300 555 0210. This leaflet has been made available to your GP, who may ask you to try the advice and exercises prior to physiotherapy assistance. About the Shoulder A. Shoulder Rolls The shoulder is the most mobile joint in the body. The main shoulder joint is a 1. Sit or stand. ball and socket joint, which allows a wide range of movement. The joint is surrounded by a tough fibrous sleeve called the capsule, which helps to support 2. Roll your shoulders backwards. the joint. A group of four muscles and their tendons make up the rotator cuff, which 3. Repeat up to 10-15 times. Frequency: 2-3 times per day. controls movement and also helps to support the joint. There’s another smaller joint where the top of the shoulder blade meets the collarbone. -

Shoulder Pain: Differential Diagnosis with Mechanical Diagnosis and Therapy Extremity Assessment E a Case Report
Manual Therapy 18 (2013) 354e357 Contents lists available at SciVerse ScienceDirect Manual Therapy journal homepage: www.elsevier.com/math Case report Shoulder pain: Differential diagnosis with mechanical diagnosis and therapy extremity assessment e A case report A. Menon a,S.Mayb,* a Shri Giridhari Physical Therapy Center, Mumbai, India b Faculty of Health and Wellbeing, 38 Collegiate Crescent Campus, Sheffield Hallam University, Sheffield S10 2BP, UK article info abstract Article history: Mechanical Diagnosis and Therapy (MDT) is now probably the only non-specific classification system Received 29 May 2012 that can be used with both spinal and non-spinal patients. Derangement syndrome, which presents with Received in revised form rapid changes in symptoms or mechanical response with the use of repeated movements, appears to be 20 June 2012 common in both spinal and extremity cases. Shoulder problems can be attributed to specific shoulder Accepted 26 June 2012 problems, but may also be referred from the neck. The case report describes a patient presenting with shoulder pain and limitations to shoulder function, and who tested positive to functional shoulder tests. Keywords: However, repeated neck movements demonstrated the ability to worsen and improve the shoulder pain McKenzie ‘ ’ Mechanical diagnosis and therapy and limitations. MDT was an effective tool at making the differential diagnosis between genuine and Shoulder referred shoulder pain. Classification Ó 2012 Elsevier Ltd. All rights reserved. 1. Introduction prognosis and facilitates research (Long et al., 2004; Cook et al., 2005). Shoulder pain is reported to be the most common musculo- The McKenzie Method of Mechanical Diagnosis and Therapy skeletal disorder after spinal pain (Eltayeb et al., 2007). -

Shoulder Pain
Health News from Ponte Vedra Wellness Center Family Chiropractic Care in Ponte Vedra Beach & Nocatee Town Center Dr. Erika Hamer, DC, DIBCN, DIBE CHIROPRACTIC FROM HEAD TO TOE – SHOULDER PAIN he shoulder is a very unique and complex joint. It is the only articulation in the body whose stability is granted primarily by muscular balance instead of being held Ttogether by the integrity of its ligaments. Have you ever heard of the ROTATOR CUFF? This is the collection of four shoulder muscles that help perform this function: namely the supraspinatus, the infraspinatus, the teres minor and subscapularis. In addition, there are multiple bones that come togeth- er to make up the shoulder joint: the humerus (upper arm), the scapula (the shoulder blade) and the clavicle (the collar bone). Therefore, problems with the align- ment of any of these bones will directly aff ect shoulder function, potentially leading to dysfunction and painful symptoms in this joint. Finally, many nerves lead to the shoulder, infl uencing motor control of the shoulder and providing sensory feedback from the shoulder to the brain. The main source of these nerves is the cervical spine (the neck). How Chiropractic Helps Shoulders So, how can your chiropractor help when you have a shoulder problem? Just like every other joint in the body, the shoulder works best when all its moving parts are in proper alignment. As you will discover below, the most important factor contributing to the proper function of the shoulder is the alignment of your spine, with each major area of the spine contributing in a signifi cant way to the health of your shoulder. -

Welcome Pack
Jing Certificate in Advanced Clinical Massage WELCOME PACK JING INSTITUTE OF ADVANCED MASSAGE TRAINING PO BOX 5291, BRIGHTON BN50 8AG l VAT NO: 866374290 CONTACT US www.jingmassage.com 1 2 Table of Contents Section 1: Syllabus and summary of modules Section 2: Guidance notes for students and contract Section 3: Reading list Section 4: Notes on assessment Section 5: Structuring your study; guidelines and sample quizzes 3 4 Certificate in Advanced Clinical Massage – Student syllabus Learning Assessment Criteria Underpinning knowledge Outcome UnitIntroduction to Advanced Massage Techniques – 120 learning hours 1 1.1 Utilise 1.1.1 Evaluate Client assessment – record taking based on appropriate assessment HOPRS; basic visual assessment; range of assessment techniques based movement; pain levels techniques for on HOPRS Client communication – explanation of presenting 1.1.2 Utilise correct treatment; importance of communication & client assessment conditions techniques for feedback; basic listening & questioning based on presenting client skills; non verbal communication in HOPRS conditions assessment 1.2 Demonstrate 1.2.1 Perform all specific Techniques – effleurage; petrissage; the ability to techniques tapotement; trigger point therapy; perform all correctly on neuromuscular techniques; muscle energy specific superficial muscles technique; myofascial release including techniques 1.2.2 Perform all specific indirect and direct fascial approaches, correctly and techniques appropriately appropriately using structural integration, basics of craniosacral -

WCAT Decision 2005-02580
WCAT Decision Number: WCAT-2005-02580 NOTEWORTHY DECISION SUMMARY Decision: WCAT-2005-02580 Panel: Joanne Kembel Decision Date: May 19, 2005 Pre-existing Conditions – Shoulder Dislocation – Wage Loss Benefits – Section 29(1) of the Workers Compensation Act – Section 30(1) of the Act – Policy Item #15.60 of the Rehabilitation Services and Claims Manual, Volume II (RSCM II) This case is noteworthy as an example of the application of those portions of policy item #15.60 of the Rehabilitation Services and Claims Manual, Volume II (RSCM II) which provide rules for the payment of benefits in shoulder dislocation claims where the worker has previously experienced a non-compensable shoulder dislocation. Item #15.60 of the RSCM II provides that if a worker has a prior non-compensable shoulder dislocation and suffers another dislocation at work, the later dislocation will only be compensable if there was a work incident of “sufficient causative significance to induce the further dislocation”. Once the claim is accepted the policy provides rules in respect of the payment of benefits. The policy provides, firstly, that temporary wage loss benefits will “normally endure no more than two weeks,” and secondly, that any surgery, after the injury, which is directed at repairing damage from the prior dislocation will not be compensable except in cases where: a) the shoulder has been stable for many years prior to the dislocation at work, or b) the dislocation at work was induced by “severe trauma.” In this case, the worker dislocated his left shoulder. The worker had a long history of left shoulder dislocations, and had surgery on his left shoulder three years earlier. -

SHOULDER PROBLEMS Yourphysio Ongoing Needs
SPRING 2011 Q: I suffer from back pain. Q: My mother fractured her wrist. The plaster has just 25 Wantirna Road What should I do? come off. When should she start physio? RINGWOOD25 Wantirna VIC Road 3134 PHRINGWOOD (03) 9870 VIC 8193 3134 A: Have your back problem www.physica.com.auPH (03) 9870 8193 assessed by a physio as soon as SPRING 2011 you can. Treatments such as manual therapy (to free up stiff and Dear Patient, www.physica.com.auHOURS painful spinal joints), mobilizing exercises and exercises aimed at WelcomeDear Patient,to our newsletter and The practiceHOURS hours are strengthening your core muscles can help a lot of back problems. Medications such as pain killers and anti-inflammatory can thanksWelcome for comingto our newsletter to see us for and your physio needs. MON-FRI:The practice 7.00am hours - 8.00pm are be useful but don’t actually fix the problem. Don’t rely on thanks for coming to see us for your SHOULDER PROBLEMS Yourphysio ongoing needs. health is very important MON-FRI:SAT: 8.00am 7.00am - 1pm- 8.00pm theses passive measures. Take control by consulting your A: Start physio straight away. Your mother needs to get the physiotherapist. wrist moving and to regain strength as soon as possible so that toYour all ofongoing us here health at the is clinic. very importantWe hope (Please SAT:ring for8.00am an appointment) - 1pm See your physiotherapist for a quick recovery she can get back to normal activity. Leaving physio off for too thatto all this of newsletterus here at thewill clinic.help keep We hopeyou (Please ring for an appointment) up to date with information about Q: I sit at a desk all day. -

Shoulder Instability Anatomy
Shoulder Instability The shoulder is your body’s most flexible joint. It is designed to let the arm move in almost any direction. But this flexibility has a price, making the joint prone to injury. The shoulder is made up of bones, muscles, ligaments, and tendons. They work together so you can comfortably reach, swing, lift, and throw a ball. Learning about the parts of the shoulder will help you understand your shoulder problem. Anatomy Bones provide the foundation of the shoulder joint. The bones fit together in a way that allows the arm to move freely. • The humeral head is the ball at the top of the humerus (arm bone). • The glenoid is the shallow socket located on the scapula (shoulder blade). • The labrum is a ring of cartilage around the rim of the glenoid. Important ligaments attach to the labrum and connect to the humerus. The labrum and these ligaments provide stability to the shoulder joint. • The coracoid and acromion are two other parts of the scapula. Muscles attach on these structures. • The clavicle is the collar bone. The clavicle connects to the acromion, forming the acromioclavicular (AC) joint. Soft tissues include muscles, tendons, and ligaments. These connect the shoulder bones together, provide stability, and movement to the joint. • The capsule is a sheet of tough fibers that encloses the joint. The glenohumeralligaments are thickened parts of the Capsule capsule that connect the humerus to the labrum. The labrum is firmly attached to the rim of the glenoid. The capsule, ligaments, and labrum provide most of the stability to the shoulder joint. -
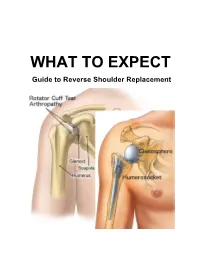
Guide to Reverse Shoulder Replacement TABLE of CONTENTS
WHAT TO EXPECT Guide to Reverse Shoulder Replacement TABLE OF CONTENTS 1. Introduction Welcome Letter Key Contacts Our Neighborhood 2. Surgery Preparation What is a Reverse Shoulder Replacement? Pre-Operation Checklist Planning Your Hospital Stay 3. Day of Surgery What to Expect on the Day of Surgery Your Anesthesiologist and Anesthesia Blood Transfusions 4. Initial Recovery in Post Anesthesia Care Unit Overview Relaxation Exercises Pain Management 5. Recovery & Rehabilitation Overview of Post-Operative Recovery Prevention of Post-Operative Complications Anticoagulation Therapy & Thrombosis Rehabilitation Overview 2 TABLE OF CONTENTS (continued) 6. Progress Guidelines Post Reverse Shoulder Replacement 7. Discharge Instructions Surgical Site Care Pain Management Protection Against Infection When Can You Begin Driving? Follow Up Appointments 8. Home Recovery & Exercise General Recovery Guidelines Bathing/Showering Dressing Rehabilitation 9. Nutrition Nutrition Before Surgery Nutrition on Day of Surgery Nutrition During Hospital Stay Nutrition After Discharge Food Guide Pyramid My Meal Plan: 1200 Calories 1800 Calories 2200 Calories . 10. Other Pastoral Care Other Educational Resources 3 Dear Patient, Welcome to Covenant Healthcare. In an effort to help you get the most out of your hospital experience, we have developed this guide to help you before, during, and after your hospital stay. The objectives of this guide are: 1) To help prepare you for your surgery and hospital experience 2) To optimize your recovery from your Reverse Shoulder Replacement while in the hospital and later at home. It is important to remember that this is only a general guide to recovery from your surgery. Keep in mind that not all patients have the same medical conditions or needs. -

United States District Court Eastern District of Tennessee at Chattanooga
UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TENNESSEE AT CHATTANOOGA ANTHONY RAY JOHNSON, ) ) Plaintiff, ) ) No. 1:12-cv-66 v. ) ) Collier / Lee COMMISSIONER OF SOCIAL SECURITY, ) ) Defendant. ) REPORT AND RECOMMENDATION Plaintiff Anthony Ray Johnson brought this action pursuant to 42 U.S.C. §§ 405(g) seeking judicial review of the final decision of the Commissioner of Social Security (“Commissioner” or “Defendant”) denying him disability insurance benefits (“DIB”). Plaintiff has moved for judgment on the pleadings [Doc. 10] and Defendant has moved for summary judgment [Doc. 12]. Plaintiff alleges the Administrative Law Judge (“ALJ”) failed to evaluate the evidence to determine whether Plaintiff met a Listing, did not adequately address his impairments in combination, and did not pose an appropriate hypothetical question to the vocational examiner (“VE”). For the reasons stated below, I RECOMMEND that (1) Plaintiff’s motion for judgment on the pleadings [Doc. 10] be GRANTED IN PART to the extent it seeks remand to the Commissioner and DENIED IN PART to the extent it seeks reversal and an award of benefits; (2) the Commissioner’s motion for summary judgment [Doc. 12] be DENIED; and (3) the Commissioner’s decision denying benefits be REVERSED and REMANDED pursuant to Sentence Four of 42 U.S.C. § 405(g). I. ADMINISTRATIVE PROCEEDINGS Plaintiff initially filed his application for DIB on July 2, 2009, alleging disability as of May 21, 2008 (Transcript (“Tr.”) 131-33). Plaintiff’s claim was denied initially and upon reconsideration Case 1:12-cv-00066-CLC-SKL Document 14 Filed 02/28/13 Page 1 of 32 PageID #: <pageID> and he requested a hearing before the ALJ (Tr. -

Joint Pain Advisory Clinic (JPAC) Shoulder Pain
Joint Pain Advisory Clinic (JPAC) Shoulder Pain healthshare.org.uk 1 2 Joint Pain Advisory Clinic - Shoulder Pain Joint Pain Advisory Clinic (JPAC) Contents The Joint Pain Advisory Clinic 2 Your shoulder pain 2 Understanding the shoulder 5 Pain 7 Do I need a scan? 11 Start feeling better with these options 12 Exercise advice 13 Exercises 14 What else can help your pain 15 Healthier lifestyles 16 Making changes 19 Goal setting 20 Barriers to change 22 Breaking barriers 23 Injections and surgery 24 Useful websites and your local information 25 1 Joint Pain Advisory Clinic for Shoulder Pain The Joint Pain Advisory Clinic, or JPAC for short, is our way This booklet is designed specifically for those people of getting you to the right clinician, at the right time, first time. suffering from shoulder pain. For more information we recommend you watch our JPAC video on our website. Pain is unique and individual to you. During the JPAC you will meet with others who are experiencing similar problems The JPAC makes use of the latest research for you to learn which can help in supporting you during your ongoing about your injury, explore self-care techniques and discuss treatment. For us to understand your experience to date we the problems which may be contributing to your pain. suggest you work through this booklet before you come to the JPAC. Your Shoulder Pain Status on shoulder pain (Circle, tick or write) Typical age range Less than 18 | 18-40 | and over Location of pain Front | top | down arm Type of pain Sharp | dull | ache | catching | shooting -

Total Shoulder Replacement Handbook
NAON Patient Education Series Postoperative Shoulder Copyright © 2013 by National Association of Orthopaedic Nurses. All rights reserved. This publication, in its entirety or specific pages, is intended to be printed and distributed as needed to patients undergoing Shoulder Replacement. Content may not be copied and reproduced without written permission of the National Association of Orthopaedic Nurses. National Organization of Orthopaedic Nurses 330 N. Wabash Avenue, Suite 2000 Chicago, IL 60611 Phone: 800.289.6266 E-mail: [email protected] Website: www.orthonurse.org Postoperative Shoulder – National Association of Orthopaedic Nurses Made possible with sponsorship by Pacira Pharmaceuticals, Inc. is an emerging specialty pharmaceutical company focused on the clinical and commercial development of new products to address the needs of acute care practitioners and their patients. Pacira is driven by a dynamic workforce committed to optimizing patient care and satisfaction in the acute care setting, with a special focus on improving outcomes in postsurgical pain management. Website: www.exparel.com/patient Postoperative Shoulder – National Association of Orthopaedic Nurses Multimodal Analgesia Local Anesthetic Injection Pain is generated from multiple nerve pathways in your One important part of multimodal analgesia (see above for body. To ensure the best possible pain relief after shoulder an explanation of this term) for pain following surgery is surgery, your doctors may use a pain control approach local anesthetic injection. Your surgeon may use this called multimodal analgesia. Multimodal analgesia means procedure during your shoulder surgery. The surgeon that you will receive two or more medications that provide injects a local anesthetic (similar to novocaine given at the pain relief and, when used together, more effectively block dentist) alone or in combination with other medications into pain signals. -

Inside... Shoulder”—Without Drugs Or a Word from Restore Normal Surgery ! Dr
Your photo • here Vol. 24, Issue 7 Personalized salutation • with actual birthday and personal message! • Your (optional) • offer or • birthday gift Your patient’s at NO COST name on the cake! TO YOU!! Personal salutation You depend on your shoulders turn into “frozen shoulder”—where practically every hour of every day. your joint is so excruciatingly That’s why maintaining “pain- painful you cannot move it in any When you refer any professional in Dentistry, Chiropractic or free” shoulder motion should direction. “OUCH ! ! !” would be an Mortgage that signs up and mails any of our newsletters, we’ll always be a priority. understatement! credit your group 1 account for your next month’s print fee! The Risk of Ignoring Please don’t neglect your pain to Your “Achy Shoulder”! the point that you need surgery or You could end up getting your until a surgeon has to aggressively The longer you ignore your stretch your shoulder joint under entire year’s printing... symptoms, the greater the chance general anesthesia to break up FREE!! hether you’re reaching of faulty shoulder function and your scar tissue to restore normal This does not apply to mortgage brokers or insurance up to fix your hair, formation of scar tissue, which movement. That’s exactly what can agents when they refer someone in their own office. W handling heavy work further restricts joint motion. happen when you neglect a loads, or just hanging up your shoulder problem ! Dental- Patient Education Newsletters to maintain retention coat, life can be hard when your Worse yet, what