Federal Register/Vol. 85, No. 28/Tuesday, February
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thursday, February 11, 2020 - Summary Minutes
(approved by committee 3/12/2020) RACINE COUNTY PUBLIC WORKS, PARKS AND FACILITIES COMMITTEE MEETING THURSDAY, FEBRUARY 11, 2020 - SUMMARY MINUTES (NOTE: Audio recording from Racine County is available upon request. These minutes are intended as a summary of actions taken by the Committee and do not include verbatim or detailed accounts of all comments made by staff, the Committee and members of the general public present at the meeting.) Public Works-Development Services Conference Room Ives Groves Office Complex 14200 Washington Avenue Sturtevant, WI 53177 __________________________________________________________________________________ Committee Present: Robert Grove, Mike Dawson, Tom Kramer, Nick Demske Committee Excused: Monte G. Osterman, Fabi Maldonado, Tom Hincz Youth in Governance Representatives Present: Katlynn Gill, Jillian Humphreys Staff Present: Julie Anderson, Public Works & Development Services Director Dave Prott, Highway Superintendent David Cooke, County Board Vice-Chair Don Trottier, County Board Supervisor Jonathan Delagrave, Racine County Executive __________________________________________________________________________________ 1. Call to Order, Roll Call The meeting was called to order at 5:00 p.m. by Chairman Robert Grove. 2. Review and possible approval of the January 23, 2020, summary minutes SUPERVISOR Tom KRAMER MOVED, seconded by Supervisor DAWSON, to approve the January 23, 2020, summary minutes as presented. YOUTH REPRESENTATIVES' ADVISORY VOTES: Youth Representative Gill: aye Youth Representative Humphreys: aye Motion carried unanimously. VOTE: 4/0 Racine County Public Works, Parks and Facilities Committee Meeting Ives Grove Office Complex, Public Works Development Services Conference Room February 11, 2020, Summary Minutes Page 2 of 3 3. Discussion regarding the status of Caledonia-Mt Pleasant Memorial Park in Franksville. Anderson, Prott and Co Exec Delagrave informed the Committee about the status of the Caledonia-Mt Pleasant Park. -
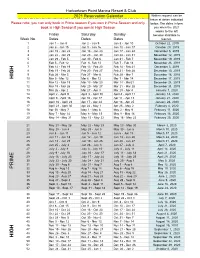
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

2018 - 2019 Days of Rotation Calendar
2018 - 2019 DAYS OF ROTATION CALENDAR Day # Date Rotation Day Type Notes Day # Date Rotation Day Type Notes Saturday, October 13, 2018 Sunday, October 14, 2018 Monday, September 3, 2018 Holiday/Vaca Labor Day 27 Monday, October 15, 2018 Day 3 In Session 1 Tuesday, September 4, 2018 Day 1 In Session 28 Tuesday, October 16, 2018 Day 4 In Session 2 Wednesday, September 5, 2018 Day 2 In Session 29 Wednesday, October 17, 2018 Day 5 In Session 3 Thursday, September 6, 2018 Day 3 In Session 30 Thursday, October 18, 2018 Day 6 In Session 4 Friday, September 7, 2018 Day 4 In Session 31 Friday, October 19, 2018 Day 1 In Session Saturday, September 8, 2018 Saturday, October 20, 2018 Sunday, September 9, 2018 Sunday, October 21, 2018 Monday, September 10, 2018 Day Holiday/Vaca Rosh Hashanah 32 Monday, October 22, 2018 Day 2 In Session 5 Tuesday, September 11, 2018 Day 5 In Session 33 Tuesday, October 23, 2018 Day 3 In Session 6 Wednesday, September 12, 2018 Day 6 In Session 34 Wednesday, October 24, 2018 Day 4 In Session 7 Thursday, September 13, 2018 Day 1 In Session 35 Thursday, October 25, 2018 Day 5 In Session 8 Friday, September 14, 2018 Day 2 In Session 36 Friday, October 26, 2018 Day 6 In Session Saturday, September 15, 2018 Saturday, October 27, 2018 Sunday, September 16, 2018 Sunday, October 28, 2018 9 Monday, September 17, 2018 Day 3 In Session 37 Monday, October 29, 2018 Day 1 In Session 10 Tuesday, September 18, 2018 Day 4 In Session 38 Tuesday, October 30, 2018 Day 2 In Session Wednesday, September 19, 2018 Day Holiday/Vaca Yom Kippur 39 Wednesday, October 31, 2018 Day 3 In Session 11 Thursday, September 20, 2018 Day 5 In Session 40 Thursday, November 1, 2018 Day 4 In Session 12 Friday, September 21, 2018 Day 6 In Session 41 Friday, November 2, 2018 Day 5 In Session Saturday, September 22, 2018 Saturday, November 3, 2018 Sunday, September 23, 2018 Sunday, November 4, 2018 13 Monday, September 24, 2018 Day 1 In Session 42 Monday, November 5, 2018 Day 6 In Session 14 Tuesday, September 25, 2018 Day 2 In Session Tuesday, November 6, 2018 Prof Dev. -
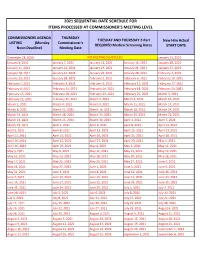
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

Federal Register/Vol. 85, No. 28/Tuesday, February
7816 Federal Register / Vol. 85, No. 28 / Tuesday, February 11, 2020 / Notices requested by the Commission is All comments received will be posted Partnership Option received, if the Commission notifies the without change. Persons submitting CONTACT PERSON FOR MORE INFORMATION: clearing agency in writing that it does comments are cautioned that we do not For more information: Please call Jim not object to the proposed change and redact or edit personal identifying Hopson, TVA Media Relations at (865) authorizes the clearing agency to information from comment submissions. 632–6000, Knoxville, Tennessee. People implement the proposed change on an You should submit only information who plan to attend the meeting and earlier date, subject to any conditions that you wish to make available have special needs should call (865) imposed by the Commission. publicly. 632–6000. Anyone who wishes to OCC shall post notice on its website All submissions should refer to File comment on any of the agenda in of proposed changes that are Number SR–OCC–2020–801 and should writing may send their comments to: implemented. be submitted on or before February 26, TVA Board of Directors, Board Agenda The proposal shall not take effect 2020. Comments, 400 West Summit Hill until all regulatory actions required By the Commission. Drive, Knoxville, Tennessee 37902. with respect to the proposal are J. Matthew DeLesDernier, Dated: February 6, 2020. completed. Assistant Secretary. Sherry A. Quirk, IV. Solicitation of Comments [FR Doc. 2020–02622 Filed 2–10–20; 8:45 am] General Counsel. Interested persons are invited to BILLING CODE 8011–01–P [FR Doc. -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November -

2021 Working Day Calendar-5 Day-Alternative Format
2021 Working Days Calendar – 5 day The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 Non-working Day Saturday, January 2, 2021 Non-working Day Sunday, January 3, 2021 Non-working Day Monday, January 4, 2021 938 Tuesday, January 5, 2021 939 Wednesday, January 6, 2021 940 Thursday, January 7, 2021 941 Friday, January 8, 2021 942 Saturday, January 9, 2021 Non-working Day Sunday, January 10, 2021 Non-working Day Monday, January 11, 2021 943 Tuesday, January 12, 2021 944 Wednesday, January 13, 2021 945 Thursday, January 14, 2021 946 Friday, January 15, 2021 947 Saturday, January 16, 2021 Non-working Day Sunday, January 17, 2021 Non-working Day Monday, January 18, 2021 Non-working Day Tuesday, January 19, 2021 948 Wednesday, January 20, 2021 949 Thursday, January 21, 2021 950 Friday, January 22, 2021 951 Saturday, January 23, 2021 Non-working Day Sunday, January 24, 2021 Non-working Day Monday, January 25, 2021 952 Tuesday, January 26, 2021 953 Wednesday, January 27, 2021 954 Thursday, January 28, 2021 955 Friday, January 29, 2021 956 Saturday, January 30, 2021 Non-working Day Sunday, January 31, 2021 Non-working Day Monday, February 1, 2021 957 Tuesday, February 2, 2021 958 Wednesday, February 3, -

Date of Pascha (New Calendar)
Date of Pascha (New Calendar) Pub&Pharisee Meatfare Cheesefare First Day of Lent Pascha Ascenion Pentecost 2007 January 28, 2007 February 11, 2007 February 18, 2007 February 19, 2007 April 8, 2007 May 17, 2007 May 27, 2007 2008 January 13, 2008 January 27, 2008 February 3, 2008 February 4, 2008 March 23, 2008 May 1, 2008 May 11, 2008 2009 February 1, 2009 February 15, 2009 February 22, 2009 February 23, 2009 April 12, 2009 May 21, 2009 May 31, 2009 2010 January 24, 2010 February 7, 2010 February 14, 2010 February 15, 2010 April 4, 2010 May 13, 2010 May 23, 2010 2011 February 13, 2011 February 27, 2011 March 6, 2011 March 7, 2011 April 24, 2011 June 2, 2011 June 12, 2011 2012 January 29, 2012 February 12, 2012 February 19, 2012 February 20, 2012 April 8, 2012 May 17, 2012 May 27, 2012 2013 January 20, 2013 February 3, 2013 February 10, 2013 February 11, 2013 March 31, 2013 May 9, 2013 May 19, 2013 2014 February 9, 2014 February 23, 2014 March 2, 2014 March 3, 2014 April 20, 2014 May 29, 2014 June 8, 2014 2015 January 25, 2015 February 8, 2015 February 15, 2015 February 16, 2015 April 5, 2015 May 14, 2015 May 24, 2015 2016 January 17, 2016 January 31, 2016 February 7, 2016 February 8, 2016 March 27, 2016 May 5, 2016 May 15, 2016 2017 February 5, 2017 February 19, 2017 February 26, 2017 February 27, 2017 April 16, 2017 May 25, 2017 June 4, 2017 2018 January 21, 2018 February 4, 2018 February 11, 2018 February 12, 2018 April 1, 2018 May 10, 2018 May 20, 2018 2019 February 10, 2019 February 24, 2019 March 3, 2019 March 4, 2019 April 21, 2019 -

February 11, 2021 Conference Call/Virtual Meeting
NATURAL RESOURCES COMMISSION MEETING MINUTES February 11, 2021 Conference Call/Virtual Meeting Present for the Natural Resources Commission (NRC) Carol Moncrieff Rose, Chair, Hillman, MI David Nyberg, Marquette County, MI J.R. Richardson, Carp Lake Township, MI Keith Creagh, Williamston Township, MI Mike Lashbrook, East Lansing, MI Present for Department of Natural Resources (Department) Staff Dan Bock, Legal Counsel, Office of the Attorney General, Lansing, MI Daniel Eichinger, DNR Director, Isabella County, MI Trevor VanDyke, DNR Director of Legal and Legislative Affairs Office Brooke Parmalee, DNR, Legal and Legislative Affairs Office Cheryl Nelson, Executive Assistant to the NRC Chair Rose called the meeting of the NRC to order at 9:00 a.m. Chair Rose asked for a roll call of the Commissioners; all Commissioners, Director Eichinger and Assistant Attorney General Dan Bock, were present virtually for the February 11, 2021, NRC meeting. Chair Rose then called for a motion to approve the January 14, 2021, and the February NRC meeting agenda. Chair Rose indicated that a new item pertaining to an NRC Resolution would be added under New Business. Commissioner Lashbrook made a motion to adopt the day’s agenda; Commissioner Nyberg supported the motion. Chair Rose called for discussion, there being none, a vote was taken, and the agenda was approved unanimously. The next item on the agenda was the approval of the January 14, 2021, NRC meeting minutes. Chair Rose called for a motion to approve the minutes; Commissioner Lashbrook made the motion to adopt the January 14, 2021 minutes, Commissioner Nyberg seconded the motion. Chair Rose then called for additional discussion, there being none, a vote was taken, and the January 14, 2021, meeting minutes passed unanimously.