+1.518.758.8158
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2015-02 Toxicology Rapid Testing Panel
SOUTH CAROLINA LAW ENFORCEMENT DIVISION NIKKI R. HALEY MARK A. KEEL Governor Chief FORENSIC SERVICES LABORATORY CUSTOMER NOTICE 2015-02 REGARDING TOXICOLOGY RAPID TESTING PANEL August 12, 2015 This notice is to inform the Coroners of South Carolina of a new testing panel available through the SLED Toxicology Department. On Monday, August 17th, the Toxicology Department will begin offering both a Rapid Testing Panel in addition to the already available Expanded Testing Panel. This Rapid Testing Panel is to be utilized in cases where the Expanded Testing Panel is not warranted, specifically where a cause of death has already been established. The Rapid Testing Panel will consist of volatiles analysis, to include, ethanol, acetone, isopropanol and methanol, drug screens, and drug confirmation/quantitation of positive screens. The cases assigned to the Rapid Testing Panel will have an expedited turnaround time. Targeted turn around times will be two weeks for negative cases and six weeks or less for positive cases. While every effort will be made to adhere to these time frames, additional time may be required on occasion due to the nature of postmortem samples. Submitters will be notified if there is a problem with a particular sample. Please see attachment regarding specifically which substances are covered by the Rapid Testing Panel and the Expanded Testing Panel. As always, a detailed case history and list of drugs suspected is appreciated. Rapid Panel and Expanded Panel will be choices available in iLAB. Please contact Lt. Dustin Smith (803-896-7385) with additional questions. ALI-359-T An Accredited Law Enforcement Agency P.O. -

Drug-Facilitated Sexual Assault Panel, Blood
DRUG-FACILITATED SEXUAL ASSAULT PANEL, BLOOD Blood Specimens (Order Code 70500) Alcohols Analgesics, cont. Anticonvulsants, cont. Antihistamines, cont. Ethanol Phenylbutazone Phenytoin Cyclizine Amphetamines Piroxicam Pregabalin Diphenhydramine Amphetamine Salicylic Acid* Primidone Doxylamine BDB Sulindac* Topiramate Fexofenadine Benzphetamine Tapentadol Zonisamide Guaifenesin Ephedrine Tizanidine Antidepressants Hydroxyzine MDA Tolmetin Amitriptyline Loratadine MDMA Tramadol Amoxapine Oxymetazoline* Mescaline* Anesthetics Bupropion Pyrilamine Methcathinone Benzocaine Citalopram Tetrahydrozoline Methamphetamine Bupivacaine Clomipramine Triprolidine Phentermine Etomidate Desipramine Antipsychotics PMA Ketamine Desmethylclomipramine 9-hydroxyrisperidone Phenylpropanolamine Lidocaine Dosulepin Aripiprazole Pseudoephedrine Mepivacaine Doxepin Buspirone Analgesics Methoxetamine Duloxetine Chlorpromazine Acetaminophen Midazolam Fluoxetine Clozapine Baclofen Norketamine Fluvoxamine Fluphenazine Buprenorphine Pramoxine* Imipramine Haloperidol Carisoprodol Procaine 1,3-chlorophenylpiperazine (mCPP) Mesoridazine Cyclobenzaprine Rocuronium Mianserin* Norclozapine Diclofenac Ropivacaine Mirtazapine Olanzapine Etodolac Antibiotics Nefazodone Perphenazine Fenoprofen Azithromycin* Nordoxepin Pimozide Hydroxychloroquine Chloramphenicol* Norfluoxetine Prochlorperazine Ibuprofen Ciprofloxacin* Norsertraline Quetiapine Ketoprofen Clindamycin* Nortriptyline Risperidone Ketorolac Erythromycin* Norvenlafaxine Thioridazine Meclofenamic Acid* Levofloxacin* Paroxetine -

A Complete Toxicology Screening Procedure for Drugs and Toxic Compounds in Urine and Plasma Using LC-MS/MS
Application Note: 449 A Complete Toxicology Screening Procedure for Drugs and Toxic Compounds in Urine and Plasma Using LC-MS/MS Marta Kozak, Taha Rezai, Thermo Fisher Scientific, San Jose, CA Introduction Key Words Toxicology laboratories commonly use automated Scan Event 1 Scan Event 7 + Full Scan MS – Full Scan MS • ToxSpec immunoassays, gas chromatography-mass spectrometry Analyzer (GC-MS) and high pressure liquid chromatography-diode array detector (HPLC-DAD) techniques to perform • ToxID Software toxicology screening analyses. None of these techniques • LXQ Linear Ion are able to identify all the drugs and toxic compounds Trap that are potentially present in a sample. Implementation of liquid chromatography-mass spectrometry (LC-MS) for • Clinical toxicology screening provides specific and sensitive Toxicology analysis of drugs and toxic substances. The benefits of the • General LC-MS/MS screening methodology include a simple Unknown sample preparation procedure, ease of adding new Screening compounds to the screening method and fewer limitations based on compound volatility and thermal stability. In Scan Event 2-6 Scan Event 8-9 addition, Thermo Scientific ToxID automated toxicology + MS/MS on parent list – MS/MS on parent list screening software is able to automatically generate both Summary and Long Reports, avoiding the need for Figure 1: MS scan events manual analysis of each sample chromatogram. This application note describes the use of the Thermo Scientific LXQ ion trap mass spectrometer equipped with an ESI source and HPLC for identification of unknown compounds in human urine and human plasma. Step 1: Extract analytes from urine or plasma with SPE procedure Goal To develop a complete LC-MS/MS screening methodology which includes a sample preparation method, LC-MS Step 2: Analyze the samples method, spectra library, and data processing and reporting with LC-MS/MS method software. -

Analytical Reference Standards
Cerilliant Quality ISO GUIDE 34 ISO/IEC 17025 ISO 90 01:2 00 8 GM P/ GL P Analytical Reference Standards 2 011 Analytical Reference Standards 20 811 PALOMA DRIVE, SUITE A, ROUND ROCK, TEXAS 78665, USA 11 PHONE 800/848-7837 | 512/238-9974 | FAX 800/654-1458 | 512/238-9129 | www.cerilliant.com company overview about cerilliant Cerilliant is an ISO Guide 34 and ISO 17025 accredited company dedicated to producing and providing high quality Certified Reference Standards and Certified Spiking SolutionsTM. We serve a diverse group of customers including private and public laboratories, research institutes, instrument manufacturers and pharmaceutical concerns – organizations that require materials of the highest quality, whether they’re conducing clinical or forensic testing, environmental analysis, pharmaceutical research, or developing new testing equipment. But we do more than just conduct science on their behalf. We make science smarter. Our team of experts includes numerous PhDs and advance-degreed specialists in science, manufacturing, and quality control, all of whom have a passion for the work they do, thrive in our collaborative atmosphere which values innovative thinking, and approach each day committed to delivering products and service second to none. At Cerilliant, we believe good chemistry is more than just a process in the lab. It’s also about creating partnerships that anticipate the needs of our clients and provide the catalyst for their success. to place an order or for customer service WEBSITE: www.cerilliant.com E-MAIL: [email protected] PHONE (8 A.M.–5 P.M. CT): 800/848-7837 | 512/238-9974 FAX: 800/654-1458 | 512/238-9129 ADDRESS: 811 PALOMA DRIVE, SUITE A ROUND ROCK, TEXAS 78665, USA © 2010 Cerilliant Corporation. -

Development of a GC-MS Method for Determination of Benzodiazepine Series Drugs Mass Spectrometry Data Center Kirill Tretyakov1, Aviv Amirav2, Anzor Mikaia1
Development of a GC-MS method for determination of Benzodiazepine Series Drugs Mass Spectrometry Data Center Kirill Tretyakov1, Aviv Amirav2, Anzor Mikaia1 1National Institute of Standards and Technology, USA; 2Tel Aviv University, Tel Aviv, Israel. NOVELTY RESULTS and DISCUSSION 4 4 A GC-MS method for determination of drugs of Oxazepam (R =H) and Lorazepam (R =Cl) under EI do not show peaks of molecular ions while in the spectra of their N(1)-methyl analogs Substituents at 5-phenyl are the major donors of eliminating radicals under EI: benzodiazepine series is developed. Thermal and EI these peaks are present. Peaks of ions due to loss of water can be used for determination of M+· of Oxazepam and Lorazepam. induced decompositions of these compounds are studied However, one can suggest that these molecules are converted to quinazolines in a GC injection port, and GC retention indices and the [1] 2 spectra recorded correspond to dehydration products. It also has been established earlier that toluene or xylene solutions of 2 2 2 R R + by varying sample inlet configurations and ion source R + R + Oxazepam and Lorazepam at 110°C decompose with the formation of corresponding quinazoline aldehydes; they are the products of O O O O N N N N systems. Reliable GC retention index (GCRI) values and 4 water elimination. 3 - R mass spectral data are acquired. R 1 3 1 GC retention index values and mass spectra indicate degradation most of Oxazepam and Lorazepam molecules in a GC injection port R N 1 N R 1 R N R R N 3 CH R 3 and further water elimination in a GC column, and probably in EI source (Figure 1). -
Infographics About Synthetic Opioids
UNODC LEADING THE INTERGRATED GLOBAL RESPONSE TO THE OPIOID CRISIS P I L L A R 1 P I L L A R 2 P I L L A R 3 P I L L A R 4 P I L L A R 5 I N T E R N A T I O N A L L A W S T R E N G T H E N I N G C O U N T E R E A R L Y W A R N I N G A N D R A T I O N A L P R E S C R I B I N G A N D S T R E N G T H E N I N G A N D S U P P O R T I N G E N F O R C E M E N T O P E R A T I O N S N A R C O T I C C A P A C I T Y A N D T R E N D A N A L Y S I S A C C E S S T O O P I O I D S P R E V E N T I O N A N D T R E A T M E N T T O D I S R U P T T R A F F I C K I N G I N T E R N A T I O N A L C O O P E R A T I O N IDENTIFYING THE MOST PREVELANT, PERSISTANT AND HARMFUL SYNTHETIC OPIOIDS U N O D C 282 90 E A R L Y TOXICOLOGY COLLABORATING IN COUNTRIES W A R N I N G INFORMED THREATS LABORATORIES A D V I S O R Y ASSESSMENTS PHARMACOLOGICAL INFORMATION L A B O R A T O R I E S A N D D A T A P O I N T S L A B O R A T O R I E S D A T A P O I N T S G L O B A L S M A R T U P D A T E S 21,400+ 120 DATA POINTS FROM COUNTRIES SYNTHETIC SEDATIVE 74 OPIOIDS HYPNOTICS REPORTED MIRRORING TO UNODC SYNTHETIC E A R L Y OPIOID TRENDS W A R N I N G A D V I S O R Y B Y 2019 131% IN THE LAST * * 3 Y E A R S Note: * 2019 data collection not finalized LIST OF SYNTHETIC OPIOIDS REPORTED TO THE UNODC EWA FROM 2009-2019 S C H E D U L E D 2-Fluorofentanyl Furanylfentanyl 4-Fluorobutyrfentanyl Methoxyacetylfentanyl 4-Fluoroisobutyrfentanyl MT-45 S C H E D U L E I Acrylfentanyl Ocfentanil ( 1 9 6 1 ) AH-7921 Tetrahydrofuranylfentanyl Butyrfentanyl U-47700 S C H E D U L E I & I V Cyclopropylfentanyl -

Forensic Toxicology Scope of Testing and Detection Limits
J. RYAN MCMAHON II County Executive INDU GUPTA, MD, MPH MEDICAL EXAMINER’S OFFICE Commissioner of Health FORENSIC TOXICOLOGY LABORATORY CAROLYN H. REVERCOMB, MD, DABP ONONDAGA COUNTY HEALTH DEPARTMENT Chief Medical Examiner KRISTIE BARBA, MS, D-ABFT-FT CENTER FOR FORENSIC SCIENCES Toxicologist Forensic Toxicology Scope of Testing and Detection Limits Table of Contents QUALITATIVE ANALYSES.........................................................................................................2 Volatile Screen by GC/FID .............................................................................................................2 Carbon Monoxide by Microdiffusion..............................................................................................2 Carbon Monoxide by CO-Oximeter ................................................................................................2 Ethylene Glycol by GC/MS.............................................................................................................2 ELISA Buprenorphine by IA for Blood/Urine ................................................................................2 ELISA by IA for Blood ...................................................................................................................3 ELISA by IA for Urine....................................................................................................................4 Gamma Hydroxybutyrate (GHB) by GC/MS..................................................................................5 Gabapentin, -

Applicability of Biochip Array Technology to the Simultaneous Screening of a Broad Range of Drugs in Whole Blood
APPLICABILITY OF BIOCHIP ARRAY TECHNOLOGY TO THE SIMULTANEOUS SCREENING OF A BROAD RANGE OF DRUGS IN WHOLE BLOOD Randox Toxicology Ltd, 55 Diamond Road, Crumlin, Co Antrim BT29 4QY, United Kingdom e-mail: [email protected] Introduction Methodology Drug detection involves initial screening of samples for drugs. The screening Drugs of Abuse Ultra Array: Test menu procedure eliminates all negatives and positive results require confirmation using Amphetamine Meprobamate confirmatory methods. A multi-analytical approach, enabling the simultaneous Barbiturates Methamphetamine screening of drugs, would be advantageous to consolidate testing and increase Benzodiazepines 1 Methadone screening capacity during the drug testing process. Biochip array technology Benzodiazepines 2 Opiates enables the simultaneous detection of multiple analytes from a single sample. Buprenorphine Oxycodone 1 This study reports the applicability of a biochip array to the multiplex screening Cannabinoids Oxycodone 2 of a broad range of drugs in human whole blood, which increases the detection Cocaine metabolite (Benzoylecgonine) Phencyclidine capacity in testing settings. Dextromethorphan Tramadol Fentanyl Tricyclic antidepressants (TCAs generic) Generic Opioids Zolpidem Results Specificity/Cross-reactivity (CR) Amphetamine assay Methamphetamine assay Benzodiazepines 1 assay 1 2 3 4 5 Compound CR >20% Compound CR >20% Compound CR >20% 6 7 8 9 10 S(+)-Amphetamine* S(+)-Methamphetamine* Oxazepam* 11 12 13 14 15 16 17 18 19 20 (±)-MDA PMMA HCl Temazepam -

Screening of 300 Drugs in Blood Utilizing Second Generation
Screening of 300 Drugs in Blood Utilizing Second Generation Exactive Plus High-Resolution, Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Thermo Fisher Scientific, San Jose, CA FIGURE 2. Schematic diagram of the Exactive Plus high-resolution accurate FIGURE 5. ExactFinder processing method and database. FIGURE 7. LODs for around 490 compounds For targeted screening, ExactFinder uses parameters set in processing method to Overview mass benchtop mass spectrometer. identify and confirm the presence of compound based on database values. Figure 8 Purpose: Evaluate Thermo Scientific Exactive Plus high performance bench-top mass Compound LOD Compound LOD Compound LOD Compound LOD shows data review results for one donor sample. In this method, compounds were 1‐(3‐Chlorophenyl)piperazine 5 Deacetyl Diltiazem 5 Lormetazepam 5 Perimetazine 5 identified by accurate mass within 5 ppm and retention time. Identity was further spectrometer for drug screening of whole blood for forensic toxicology purposes. 10‐hydroxycarbazepine 5 Demexiptiline 5 Loxapine 5 Phenacetin 5 5‐(p‐Methylphenyl)‐5‐phenylhydantoin 10 Des(2‐hydroxyethyl)opipramol 5 LSD 5 Phenazone 5 confirmed by isotopic pattern and presence of known fragments. Methods: Whole blood samples were processed by precipitation with ZnSO / 6‐Acetylcodeine 100 Desalkylflurazepam 5 Maprotiline 5 Pheniramine 5 4 6‐Methylthiopurine 5 Desipramine 5 Maraviroc 5 Phenobarbital 5 Matrix effects were observed to be compound dependent and were generally within methanol. Samples were injected onto an HPLC under gradient conditions and 6‐Monoacetylmorphine 5 Desmethylcitalopram 5 MBDB 5 Phenylbutazone 5 7(2,3dihydroxypropyl)Theophylline 5 Desmethylclozapine 50 MDEA 5 Phenytoin 10 ±50%. -

Defining Element for All Anxiety
ANXIETY Lois E. Brenn eman, MS N, ANP, FNP, C - Previously characterized as the “worried well.” - Prevalence in US is 13.3% of population or 19.1 million (National Institute of Mental Health) - Predisposing factors: genetic, developmental, biochemical, experiential influences - Genetic disposition implicated esp for panic disorders - Negative health consequences esp increased risk psychopathology - Therapy becoming more precise - Exact diagnosis re type anxiety is important - Exam ple: panic disorder: use prim ary care services 3X as m uch as other patients - Cardiologists, neurologists, ER, GI specialists, repetitive work-ups - Problem likely to resolve with proper diagnosis and treatment - Society and person health costs are high - Anxiety disorders in US $65 billion (1994) - 31.8% of total cost mental disorders which includes indirect costs - $14.9 billion treatment - $49.6 for indirect costs (lo st pro ductivity) - Anxiety encompasses a constellation of symptoms - Characterized via irrational, involuntary thoughts and behavior - Classifications - General anxiety disorder (GAD) - Obsessive-compulsive disorder (OCD) - Panic disorder - Phob ias including social ph obia - Post-traumatic stress disorder - Defining element for all anxiety - Disruption of life by overt distress - Significantly reduced ability to carry out routine tasks - Personal; social - Vocational - Example: anxiety re exam-taking or public performance - Some nervousness is normal and beneficial - Decision to forfeit exam or cancel appearance signals impairment Generalizing Anxiety Disorder (GAD) - Formerly called overanxious disorder - One of more chronic and recurrent of all psychiatric illnesses - Affects 2.8% of US population or 4 million people - Primary care practice: may be as high as 10-15% - Unremitting excessive worry re: variety issues: family, health, work, etc. -
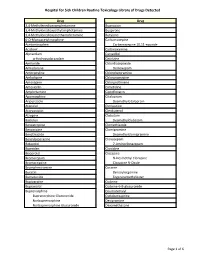
Page 1 S C Routine Toxicology Library Drug 3,4
Hospital for Sick Children Routine Toxicology Library of Drugs Detected Drug Drug 3,4-Methylenedioxyamphetamine Bupropion 3,4-Methylenedioxyethylamphetamine Buspirone 3,4-Methylenedioxymethamphetamine Butylone 6-O-Monoacetylmorphine Carbamazepine Acetaminophen Carbamazepine 10,11-epoxide Aciclovir Carbinoxamine Alprazolam Carvedilol α-Hydroxyalprazolam Cetirizine Amiloride Chlordiazepoxide Amiodarone Demoxepam Amitriptyline Chlorpheniramine Amlodipine Chlorpromazine Amoxapine Chlorprothixene Amoxicillin Cimetidine Amphetamine Ciprofloxacin Apomorphine Citalopram Aripiprazole Desmethylcitalopram Atenolol Clemastine Atorvastatin Clenbuterol Atropine Clobazam Baclofen Desmethylclobazam Benzatropine Clomethiazole Benzocaine Clomipramine Benzthiazide Desmethylclomipramine Benzylpiperazine Clonazepam Betaxolol 7-Aminoclonazepam Biperiden Clonidine Bisoprolol Clozapine Bromazepam N-Desmethyl Clozapine Bromocriptine Clozapine N-Oxide Brompheniramine Cocaine Bucetin Benzoylecgonine Bumetanide Ecgoninemethylester Bupivacaine Codeine Bupranolol Codeine-6-B-glucuronide Buprenorphine Coumatetralyl Buprenorphine Glucuronide Cyclobenzaprine Norbuprenorphine Desipramine Norbuprenorphine Glucuronide Dexamethasone Page 1 of 6 Hospital for Sick Children Routine Toxicology Library of Drugs Detected Drug Drug Dextromethorphan Fluoxetine Dextrorphan Norfluoxetine Diazepam Fluphenazine Nordiazepam Flurazepam 4-Hydroxynordiazepam Desalkylflurazepam Diclofenac 2-Hydroxyethylflurazepam Dihydrocodeine Fluvoxamine Dihydrocodeine-6-β-glucuronide Gabapentin Dihydroergotamine -

Stable Isotope Standards for Mass Spectrometry
Cambridge Isotope Laboratories, Inc. isotope.com Stable Isotope Standards For Mass Spectrometry Euriso-Top, Parc des Algorithmes, route de l'orme, 91190 Saint Aubin | France tel: +33 1 69 41 97 98 fax: +33 1 69 41 93 52 +49 (0) 681 99 63 338 (Germany) www.eurisotop.com Cambridge Isotope Laboratories, Inc. | Stable Isotope Standards for Mass Spectrometry Stable Isotope Standards for Mass Spectrometry Mass spectrometry (MS) is being increasingly implemented as The advantages of using stable isotopes include: a routine tool for scientific, translational, and clinical research. • Achieve absolute quantitation As compared to traditional standards of care in the clinical realm, • Improve accuracy and precision MS-based assays have been demonstrated to decrease errors and • Reduce analytical and technical variation lead to improved clinical outcomes.1 • Improve method transferability • Increase identification confidence Cambridge Isotope Laboratories, Inc. (CIL) recognizes the need to provide high-quality internal standards to aid in the development Reference of robust methods suitable for use in exploratory research to clinical 1. Jannetto, Paul; Fitzgerald, Robert. 2016. Effective use of mass spectrometry care. This diverse catalog of stable isotope-labeled products ranges in the clinical laboratory. Clin Chem, 62, 92-98. from small molecule standards to complete proteomic solutions. Products are manufactured and tested to meet the highest quality chemical and isotopic purity specifications commercially available, allowing researchers and clinicians to focus on what really matters – improving patient care. CIL provides additional testing on many products as a service to our customers. CIL also has cGMP capabilities and can manufacture products to meet your increasing regulatory compliance requirements.