Susan Halabi, Ph.D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chelsea Renée Singleton Curriculum Vitae
Chelsea Renée Singleton Curriculum Vitae Office Address: Contact Information: College of Applied Health Sciences Office: (217) 300-8139 2017 Khan Annex, Huff Hall, MC-588 Fax: (217) 333-2766 1206 S. Fourth Street Email: [email protected] Champaign, IL 61820 http://kch.illinois.edu/singleton Twitter: @DrCRSingleton Education 2011 – 2015 PhD Epidemiology University of Alabama at Birmingham (Birmingham, AL) Dissertation Title: “An Examination of Farm-to-Consumer Retail Outlet Usage among Participants of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) in Birmingham, AL.” Committee Chair: Olivia Affuso, PhD 2009 – 2011 MPH Epidemiology Tulane University (New Orleans, LA) Thesis Title: “Risk Factors for Severe Cerebrovascular Disease Outcomes among Chinese Adults.” 2005 – 2009 BS Biology (Pre-Med) Xavier University of Louisiana (New Orleans, LA) Cum Laude Professional Experience 2018 – Present Assistant Professor Department of Kinesiology and Community Health College of Applied Health Sciences University of Illinois at Urbana-Champaign (Champaign, IL) 2015 – 2018 USDA AFRI Post-Doctoral Research Fellow Institute for Health Research and Policy Illinois Prevention Research Center University of Illinois at Chicago (Chicago, IL) 2012 – 2015 Pre-Doctoral Research Fellow Nutrition Obesity Research Center University of Alabama at Birmingham (Birmingham, AL) Page 1 2011 – 2012 Graduate Research Assistant Department of Epidemiology University of Alabama at Birmingham (Birmingham, AL) 2010 – 2011 Data Analyst Division -

Alumni Place, Lawrence, Kansas
4, 711 11T Directory of Chapters ALPHA PHI CMEGA ALABAMA February 1957 Delta Chapter, Alabama Polytechnic Institute President -- John Brantley Smith, 2117 Magnolia Hall, API, Auburn, Alabama Gamma Chi Chapter, Howard College President -- Chriss H. Doss, Howard College, Birmingham, Alabama ARIZONA Theta Iota Chapter, University of Arizona President -- Ralph B. Miller, 1516 East 6th Street, Tucson, Arizona CALIFORNIA Zeta Chapter, Stanford University President -- John P. Hill, 616 Stern Hall, Stanford, California Chi Chapter, University of California at Los Angeles President -- Robert Dougherty, 5179 Sunset Blvd., Los Angeles 27, Calif. Psi Chapter, Santa Barbara College, University of California President -- Frank E. Wetzel, 1829 San Andres Street, Santa Barbara, Calif. Alpha Delta Chapter, San Diego State College President -- John G. Sattler, 3946 Moffet Street, San Diego 10, California Alpha Kappa Chapter, University of Southern California President -- John Leslie Jones, Jr., 1070 Glen Oaks Blvd., Pasadena, Calif. Gamma Beta Chapter, San Jose State College President -- Ronald S. Gilpatrick, 10310 Jerilyn Court, San Jose, Calif. Gamma Gamma Chapter, University of California President -- Ronald M. Kurtz, 3048 Madeline Street, Oakland 2, California Epsilon Chi Chapter, Los Angeles City College President -- Fonzo R. Dickerson, 3708 Montclair Street, Los Angeles, Calif. Zeta Omicron Chapter, California State Polytechnic College President -- John M. Ferguson, Box 1014, California Polytechnic, San Luis Obispo, California Iota Pi Chapter, City College of San Francisco President -- John F. Dudley, 1735 34th Avenue, San Francisco, California CALIFORNIA -2- Kappa Sigma Chapter, Sacramento State College President -- William R. Bonner, 5502 Carson Drive, Sacramento, California Lambda Mu Chapter, Los Angeles State College President -- Walter Clark Bauer, 6327 Hood Avenue, Huntington Park, Calif. -

UNIVERSITY CHAPTER Ashland University Epsilon Alpha Austin
The chapters listed below took in their full complement (3% of FSL community), or more than 75 new members during the 2017-2018 academic year, and are eligible to have 3 members apply for our Fall scholarship awards. Please contact HQ with any questions about this information. UNIVERSITY CHAPTER Ashland University Epsilon Alpha Austin College Alpha Alpha Delta Baker University Upsilon Tau Baylor University Theta Lambda Birmingham-Southern College Eta Xi Bridgewater State University Rho Nu Bryant University Nu Beta California Polytechnic State Uni. San Luis Obispo Gamma Kappa California State Polytechnic Univ., Pomona Iota Beta California State University, Dominguez Hills Chi Theta California State University, San Bernardino Kappa Omega California State University, San Marcos Chi Lambda California University of Pennsylvania Omicron Omicron Capital University Rho Omicron Centenary College of Louisiana Tau Theta Christopher Newport University Iota Iota Clarkson University Eta Iota Clemson University Epsilon Kappa Cleveland State University Psi Sigma Colorado School of Mines Mu Theta Colorado State University Epsilon Delta Cornell University Beta Upsilon Dartmouth College Lambda Rho Delta State University Omega Tau DePaul University Chi Alpha The chapters listed below took in their full complement (3% of FSL community), or more than 75 new members during the 2017-2018 academic year, and are eligible to have 3 members apply for our Fall scholarship awards. UNIVERSITY CHAPTER Dickinson College Sigma Omicron Drake University Delta Iota Duquesne -
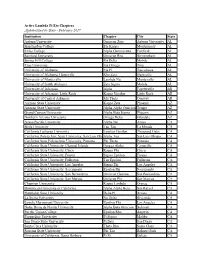
Active Lambda Pi Eta Chapters Alphabetized by State
Active Lambda Pi Eta Chapters Alphabetized by State - February 2017 Institution Chapter City State Auburn University Omicron Zeta Auburn University AL Huntingdon College Eta Kappa Montgomery AL Miles College Alpha Gamma Iota Fairfield AL Samford University Omicron Rho Birmingham AL Spring Hill College Psi Delta Mobile AL Troy University Eta Omega Troy AL University of Alabama Eta Pi Tuscaloosa AL University of Alabama, Huntsville Rho Zeta Huntsville AL University of Montevallo Lambda Nu Montevallo AL University of South Alabama Zeta Sigma Mobile AL University of Arkansas Alpha Fayetteville AR University of Arkansas, Little Rock Kappa Upsilon Little Rock AR University of Central Arkansas Mu Theta Conway AR Arizona State University Kappa Zeta Phoenix AZ Arizona State University Alpha Alpha Omicron Tempe AZ Grand Canyon University Alpha Beta Sigma Phoenix AZ Northern Arizona University Omega Delta Glendale AZ Azusa Pacific University Alpha Nu Azusa CA Biola University Tau Tau La Mirada CA California Lutheran University Upsilon Upsilon Thousand Oaks CA California Polytechnic State University, San Luis ObispoAlpha Tau San Luis Obispo CA California State Polytechnic University, Pomona Phi Theta Pomona CA California State University, Channel Islands Omega Alpha Camarillo CA California State University, Chico Kappa Phi Chico CA California State University, Fresno Sigma Epsilon Fresno CA California State University, Fullerton Tau Epsilon Fullerton CA California State University, Los Angeles Sigma Phi Los Angeles CA California State University, -

THE DELTA REPORT DELTA OMEGA CHAPTER Spring 2018 Co-Editors – Ashley Capestrain and Emily Ferstler
Sigma Theta Tau International Honor Society of Nursing THE DELTA REPORT DELTA OMEGA CHAPTER Spring 2018 Co-Editors – Ashley Capestrain and Emily Ferstler Delta Omega Officers 2017-2018 Message from the President President- Delta Omega Chapter Aris Eliades [email protected] has been busy! We have many wonderful Past President- Linda Shanks [email protected] events planned and opportunities for 1st Vice President- Chapter members to Dina Dornack [email protected] become more involved. 2nd Vice President- Lori Kidd [email protected] This newsletter is full of information from the Treasurer- 2017 Sigma Biennial Laurel Celik [email protected] Convention reported by our Chapter delegates Barb Brunt and Diane Lorenzen. President Beth Corresponding Secretary- Diane Brown [email protected] Tigges Call for Action charges us to Connect, Collaborate and Catalyze and gives us much to Faculty Counselor- consider as a Chapter and as Sigma members. Sheryl Stuck [email protected] The highlight for our chapter was receiving our 13th Key Award! Congratulations to Linda Governance Committee- Marilyn Perkowski [email protected] Shanks, immediate past president, the 2015-2017 Chapter Board of Directors and all Chapter Program Planning- members on this accomplishment! Dina Dornack [email protected] New logo and branding information was rolled Leadership Succession- Diane Lorenzen [email protected] out to Chapter leaders and members and your Delta Omega Board is transitioning to embrace Research Grants & Recognition Co-Chairs- the new Sigma brand. We are working toward Marlene Huff [email protected] moving the Chapter website from the University Barb Brunt [email protected] of Akron School of Nursing webpage to The Induction- Circle, the professional networking site of Sigma Lori Kidd [email protected] Theta Tau International. -
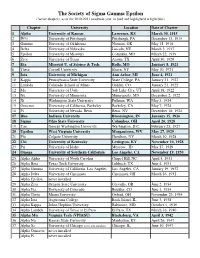
The Society of Sigma Gamma Epsilon (Active Chapters, As of the 2010-2011 Academic Year, in Bold and Highlighted in Light Blue)
The Society of Sigma Gamma Epsilon (Active chapters, as of the 2010-2011 academic year, in bold and highlighted in light blue) Chapter University Location Date of Charter 1 Alpha University of Kansas Lawrence, KS March 30, 1915 2 Beta University of Pittsburgh Pittsburgh, PA December 13, 1915 3 Gamma University of Oklahoma Norman, OK May 15, 1916 4 Delta University of Nebraska Lincoln, NE March 3, 1917 5 Epsilon University of Missouri Columbia, MO March 22, 1919 6 Zeta University of Texas Austin, TX April 30, 1920 7 Eta Missouri U. of Science & Tech. Rolla, MO January 8, 1921 8 Theta Cornell University Ithaca, NY May 10, 1921 9 Iota University of Michigan Ann Arbor, MI June 4, 1921 10 Kappa Pennsylvania State University State College, PA January 21, 1922 11 Lambda Colorado School of Mines Golden, CO January 21, 1922 12 Mu University of Utah Salt Lake City, UT April 18, 1922 13 Nu University of Minnesota Minneapolis, MN December 2, 1922 14 Xi Washington State University Pullman, WA May 3, 1924 15 Omicron University of California, Berkeley Berkeley, CA May 7, 1924 16 Pi University of Nevada, Reno Reno, NV December 18, 1924 17 Rho Indiana University Bloomington, IN January 15, 1926 18 Sigma Ohio State University Columbus, OH April 26, 1928 19 Tau George Washington University Washington, D.C. February 25, 1927 20 Upsilon West Virginia University Morgantown, WV May 27, 1929 21 Phi Colgate University Hamilton, NY March 10, 1928 22 Chi University of Kentucky Lexington, KY November 10, 1928 23 Psi University of Idaho Moscow, ID May 27, 1929 24 -

Training Location School Region Chapter BIRMINGHAM, AL – Sunday, September 15Th University of Mississippi 3 Alpha Psi Universi
Training Location School Region Chapter BIRMINGHAM, AL – Sunday, September 15th University of Mississippi 3 Alpha Psi University of Alabama 3 Beta Psi University of Georgia 3 Delta Iota University of South Florida 3 Delta Kappa Mississippi State University 3 Delta Lambda University of Southern Mississippi 3 Delta Pi Auburn University 3 Delta Sigma University of Memphis 2 Delta Zeta Loyola University 3 Epsilon Phi University of Tampa 3 Epsilon Theta Georgia College and State University n Eta Chi University of North Florida 3 Eta Delta Spring Hill College 3 Eta Eta University of Tennessee 2 Gamma Alpha Florida State University 3 Gamma Mu University of Florida 3 Gamma Theta Louisiana State University 3 Gamma Zeta Tennessee Technological University 2 Epsilon Epsilon University of Montevallo 3 Zeta Nu University of Alabama, Birmingham 3 Zeta Xi CHICAGO, IL – Saturday, September 28th Drake University 5 Alpha Lambda University of North Dakota 5 Alpha Theta Lawrence University 5 Alpha Zeta Purdue University 4 Beta Iota Northern Illinois University 5 Delta Nu Lake Forest College 5 Eta Mu DePaul University 5 Eta Pi Iowa State University n Eta Psi University of Chicago 5 Eta Zeta University of Illinois 5 Iota University of Nebraska 5 Kappa University of Minnesota 5 Lambda University of Wisconsin 5 Omega Northwestern University 5 Sigma University of Iowa 5 Tau COLUMBUS, OH – Saturday, September 14th Miami University 4 Alpha Omicron Ohio Wesleyan University 4 Alpha Rho Butler University 4 Alpha Tau West Virginia University 2 Alpha Xi Bowling Green -

AMY E. HARLEY CURRENT POSITION Associate Professor, University of Wisconsin
AMY E. HARLEY JOSEPH J. ZILBER SCHOOL OF PUBLIC HEALTH PHYSICAL: 1240 NORTH 10TH STREET, ROOM 409 MILWAUKEE, WI 53205 MAILING: P.O. BOX 413; MILWAUKEE, WI 53201-0413 PHONE: 414-227-4342 FAX: 414-227-3002 EMAIL: [email protected] CURRENT POSITION Associate Professor, University of Wisconsin - Milwaukee, Joseph J. Zilber School of Public Health 8/2016 – 8/2017, Faculty Chair 9/2014 – 9/2017, MPH Director 9/2014 – Present (Assistant Professor 9/2008 – 8/2014) Milwaukee, WI Scientist, Center for Urban Population Health 8/2008 – Present Milwaukee, WI Honorary Fellow, University of Wisconsin, School of Medicine and Public Health, Department of Population Health Sciences 12/2010 – Present Madison, WI POSTDOCTORAL TRAINING Transdisciplinary Fellow, Center for 21st Century Study 9/2013 – 12/2013 College of Letters & Science, University of Wisconsin-Milwaukee K30 Clinical Research Scholar, Clinical & Translational Science Institute of SE Wisconsin 9/2011 – 6/2013 Medical College of Wisconsin, Wauwatosa, WI Research Fellow, Harvard Education Program in Cancer Prevention and Control 9/2005 – 8/2008 Harvard School of Public Health/Dana-Farber Cancer Institute Boston, MA EDUCATION Doctor of Philosophy in Public Health 9/2000 – 6/2005 The Ohio State University Columbus, Ohio Dissertation: Physical activity evolution: A grounded theory study with African American women Master of Public Health in Community Health Education 9/1998 – 12/1999 Indiana University Bloomington, Indiana Dietetic Internship 9/1995 – 5/1996 Purdue University-Calumet Hammond, Indiana Bachelor of Science in Applied Health Science 9/1989 – 8/1995 Indiana University Bloomington, Indiana LICENSURE AND CERTIFICATION 1997 – Present Registered Dietitian Commission on Dietetic Registration HONORS AND AWARDS Community-Engaged Faculty of the Year 5/2013 Center for Community-Based Learning, Leadership, and Research University of Wisconsin-Milwaukee NIH Loan Repayment Scholar in Health Disparities 2006 - 2011 Mentor: Ron A. -

Center for Fraternity & Sorority Life
Center for Fraternity 2019 – 2020 & Sorority Life 1 WELCOME TO OREGON STATE UNIVERSITY We are excited that you chose to be a part of Beaver Nation and I know you will enjoy being an OSU student. Being in college is an amazing time to follow your educational passion/ interests while being involved in out of class experiences that complements your interests. You will get the opportunity to learn about internships, research, leadership, service projects and many other ways that will support your academic progress. Your success is our success and we are committed to you being successful at OSU. As your Dean of Students, I encourage you to connect with our office. The Office of Student Life provides educational and developmental opportunities to help students make informed decisions about your success. Our team is ready to assist you as you navigate our campus. We partner with faculty, staff, parents, and community members in our afforded effort to continue creating an environment that is welcoming where students feel a sense of belonging. Whether we are the first or last office that you visit, we are here to help you continue enjoying your experience as you persist to completing your Oregon State University degree. Member of Kappa Alpha Psi As an international public research university with students from all over the world, we are Dam Proud that you chose to pursue your education at Oregon State University! I wish you the best of luck during your time here and remember, Go Beavs! Enthusiastically, ...we are committed to Dr. Kevin A. Dougherty you being successful Associate Vice Provost & Dean Of Students at OSU. -

School of Public Health | Loma Linda, California Message from the President
ONE HUNDRED AND FOURTEENTH YEAR two thousand and twenty CONFERRING OF DEGREES School of Public Health | Loma Linda, California Message from the President Congratulations to the Class of 2020. One of the greatest joys experienced by our campus community is the opportunity to celebrate your academic excellence and personal achievements. This 114th commencement season marks the culmination of your study and professional preparation, which has equipped you to meet the next great adventures of your lives. You and those who have supported you are to be commended. Now and for all time, you occupy a place among the alumni of this historic institution. I urge you always to model in your personal and professional life the excellence and vision, the courage and resilience, the passion and compassion that continue to shape and enhance our global reputation and legacy. As you move beyond this weekend to the world of work or the pursuit of advanced degrees, I know that your commitment to our mission and values will be evident as your knowledge and skills are used to “continue the teaching and healing ministry of Jesus Christ—to make man whole.” Now go with confidence wherever your dreams may lead you—questioning, learning, and challenging as you change our world for the better. I wish for you a satisfying and successful journey as you serve in the name and spirit of our gracious God. Richard H. Hart, M.D., Dr.P.H. 1 Message from the Dean You’ve made it! You’ve completed your degree. Now begins the most important part of your learning: transforming the lives of others with your new-found knowledge, your skills, and most of all, your compassion. -

Delta Sigma 30Th Grand Chapter Congre Tan-Tar
1n• the . h professional spot 119 t ON TOUR at Chicago Pollee Headquarters are members of Zeta XI Chapter at Lewis University and members of Phi Gam· rna Nu Sorority, also at Lewis. Unidentified are pollee officials at far left and far right and the big guy In the background. 2 March, 1975 Volume LXIV, No.3 An Educational Journal • • • • • • • • • • • • • • • • • • • • • • • • • • Features Departments professional spotlight ..... .. 2 Commentary . 4 You Are Invited - to Tan-Tar-A and Mis souri for the 1975 Grand Chapter lifestyle ............. ....... 9 Congress •••• 5 kaleidoscope .... ...........33 Toward Constructive Change - What •. _ our futures may hold 26 Convention Grand Chapter Congress August 19-22, 1975 More Regional Conferences - too late Tan-Tar-A Resort for January, 1975 publication ... 34 Lake of the Ozarks, Missouri • • • • • • • • • • • • • • • • • • • • • • • • • • Cover Editor Ben H. Wolfenberger A sailboat in the sunset captures one of many moods of Lake of the Ozarks where Delta Sigma Pi will meet in August. 1975. Associate Editor Michael J. Tillar • • • • • • • • • • • • • • • • • • • • • • • • • • Postmaster: Please send labels Form 3579 to Delta Sigma Pi , 330 South Campus Avenue, Oxford, Ohio 45056. The DELTASIG of Delta Sigma Pi is published four times annually in the months of November, January, March. and May. Editorial office-330 South Campus Avenue, Oxford, Ohio 45056. Subscription price: $5.00 per year. Second Class postage paid at Oxford, Ohio 45056, and at additional mailing offices. Printed in the U.S.A. Member of College Fraternity Editors Association Commentary. • • • One might contend that each brother has been expos to a period of pledge education, but probably no t pledge education programs are exactly the sam What, then, have each of us experienced that makes brothers? Each of us, from the newest member to the old member, the honorary members of a chapter to th national honorary member-at-large, has become a member as a result of the Ritual. -

2 Table of Contents
Table of Contents Mission, Vision, and Core Values ........................................................................... 6 Strategic Goals. 2016-2020 .................................................................................... 7 ACADEMIC INFORMATION Application Requirements and Categories .............................................................. 8 Policy on Graduate Assistants .............................................................................. 11 Enrollment Policies ............................................................................................... 13 Grades .................................................................................................................. 16 Standards of Performance and Evaluation ........................................................... 18 Graduation Deadlines ........................................................................................... 21 PROFESSIONAL PROGRAMS Certificate of Population Health ............................................................................ 23 Certificate in Healthcare Administration/MBA ....................................................... 24 Master of Public Health (MPH) ............................................................................. 25 Biostatistics ............................................................................................... 32 Epidemiology ............................................................................................. 34 Health Administration and Policy .............................................................